 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Chen Yeh*
OncoDxRx, Los Angeles, CA, USA.
Correspondence to: Chen Yeh, OncoDxRx LLC, 150 N Santa Anita Ave., Suite 300, Los Angeles, CA 91006, USA.
Received date: September 02, 2024; Accepted date: September 13, 2024; Published date: September 20, 2024
Citation: Yeh C. Empowering Diagnostic Labs in Oncology: Benchmarking Biomarker-Driven Therapies. J Med Res Surg. 2024;5(5):101-103. doi: 10.52916/jmrs244146
Copyright: ©2024 Yeh C. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
In the past decade, oncology care has witnessed a transformative shift, significantly propelled by the growing field of precision medicine. This revolution has been catalyzed by a deeper understanding of cancer’s underlying biological drivers, unveiling new avenues to combat this multifaceted disease. For instance, the identification and targeting of the EGFR mutations have paved the way for personalized therapies in Non-Small Cell Lung Cancer (NSCLC).
Traditionally, diagnostic labs played a more reserved, albeit crucial, role in the healthcare continuum, primarily focusing on providing diagnostic services. However, as further insights into cancer’s molecular intricacies emerge, biomarkers have become central to the endeavor of tailoring treatments to individual patients’ genetic and molecular profiles. This rising significance of biomarkers has recalibrated the role of and expectations from diagnostic labs, ushering them into a more proactive and collaborative role in oncology care.
The shift from a one-size-fits-all approach to a more personalized, biomarker-driven therapeutic strategy in oncology has shown promise in enhancing treatment outcomes for patients and streamlining the drug development and approval processes for pharmaceutical industry. As biomarker knowledge expands, the traditional diagnostic lab has transitioned from a vendor of testing services to a strategic partner in drug development and clinical implementation.
Oncology, Precision medicine, Diagnostic labs, Biomarker, Drug development.
The dawn of biomarker-driven therapies, often encapsulated under the umbrella of precision medicine, marks a pivotal juncture in oncology. The journey begins with identifying biomarkersmolecular signatures that provide a wealth of information about a specific cancer’s genomic make-up. This has the potential to drive therapeutic decisions as well as surveillance and imaging protocols across the cancer continuum. Essentially, biomarkers are the cornerstone of precision medicine. They serve as both the roadmap and compass, guiding the development of novel therapies that can target cancer cells more accurately and with fewer side effects. A notable example is the role of the HER2 biomarker in breast cancer, which has led to the development of targeted therapies like trastuzumab [1]. From a treatment perspective, this matters immensely as it has the potential to significantly enhance the efficacy and durability of cancer treatments. Biomarker-driven therapies can pinpoint the vulnerabilities of cancer cells, enabling a more targeted assault that spares healthy cells, which is a significant departure from the collateral damage often associated with conventional chemotherapy and/or radiation. This level of precision not only holds the promise of better treatment outcomes but also heralds a new era of hope for patients and their families. Furthermore, biomarkers provide a framework for understanding the heterogeneous nature of cancer, which is crucial for developing more effective treatment strategies. They offer a more granular insight into the molecular underpinnings of each patient’s cancer, paving the way for the development of therapies that can target specific cancer subtypes or even individual tumors. Simply put, the evolution of biomarkerdriven therapies is more than just a scientific advancement; it’s a paradigm shift that positions diagnostic labs at the nexus of translating genomic insights into tangible therapeutic solutions. The precision oncology evolution also underscores a symbiotic relationship among pharmaceutical companies, diagnostic labs, and healthcare providers, each a component of a larger machinery aimed at accelerating the translation of scientific discoveries into actionable clinical solutions. The core of this alliance is to ensure precision oncology care by providing accurate molecular information in a timely manner to the treating clinician, ensuring best course of treatment for their patients.
This report will delve into the rise of biomarker-driven therapies in oncology, examining how this shift is reshaping the drug and therapy development landscape. We’ll explore the evolving role of diagnostic labs as they transition into active partners in these advancements. Through a collaborative lens, we’ll discern how precision medicine is impacting not just diagnostic labs and pharmaceutical ventures, but extending its influence to the complete patient care continuum. Our goal is to highlight the collaborative spirit emerging in oncology, shedding light on the promises and challenges that lie ahead.
The road to developing new drugs and therapies has historically been long and winding, often marked by high costs, lengthy trials, and a fair share of uncertainty. However, the foray into biomarker-driven therapies is beginning to alter the traditional narrative, offering a more streamlined and targeted approach to drug development. One of the hallmarks of biomarker-driven therapy development is the potential to significantly reduce the time to approval and increase the chances of success. A prime example of this is the accelerated approval of pembrolizumab, a PD-L1 inhibitor, for tumors with mismatch-repair deficiency irrespective of tissue origin [2]. With a clearer understanding of the molecular targets, developers can design more precise clinical trials, often requiring smaller patient cohorts to demonstrate efficacy and safety. This precision not only accelerates the regulatory approval process but also augments the chances of successful outcomes, making biomarker-driven therapies a more attractive venture for pharmaceutical companies.
Yet, this avenue is not without its considerations. A critical question that arises is whether an appropriate assay exists to identify the relevant biomarker or if a new Companion Diagnostic (CDx) needs to be developed [3]. The availability of an existing assay can expedite the drug development process, while the necessity to develop a new CDx could add an extra layer of complexity and cost. However, this challenge is often surmounted through collaborative efforts among pharmaceutical companies, regulators, and diagnostic labs, fostering a symbiotic relationship that hastens the co-development of both the drug and the companion diagnostic.
The effects of biomarker-driven therapies extend beyond the approval phase into the therapy launch and clinical uptake. The synergy between the therapeutic and diagnostic entities facilitates a smoother transition from the investigational realm to the clinical setting. It ensures that once a therapy is launched, the requisite diagnostic capabilities are already in place, promoting faster clinical adoption and broader dissemination. This integrated approach also empowers healthcare providers with the necessary tools and knowledge to prescribe these advanced therapies, bridging the gap between novel scientific discoveries and real-world clinical applications.
As precision medicine continues to gain traction in oncology, the diagnostic lab’s role is being redefined from a service provider to a strategic partner. This transition is emblematic of a broader shift towards a more collaborative, integrated approach to cancer care, where diagnostic labs, pharmaceutical companies, and healthcare providers form a triad, each contributing to the collective goal of advancing patient outcomes.
The first hallmark of this transition is the diagnostic lab’s early involvement in the drug development process. By being brought into the fold earlier, labs are better positioned to advise on the most suitable clinical trial assay or CDx for the therapy in question. This early engagement facilitates a more streamlined process towards regulatory approval and subsequent clinical uptake. Furthermore, it fosters a conducive environment for the co-development of a companion diagnostic, should one be required. This collaborative endeavor not only enhances the chances of successful therapy development but also expedites the availability of these therapies to patients in need.In the age of precision medicine, ensuring that new therapies are accessible to patients necessitates a parallel focus on diagnostic testing (Figure 1). Precise treatment is predicated on the ability to accurately identify the biomarker(s) associated with the treatment options available. Here, the diagnostic lab plays a pivotal role in ensuring that physicians are equipped with the necessary knowledge and tools to order the correct diagnostic test. This has a ripple effect, streamlining the process from diagnosis to treatment decision-making.
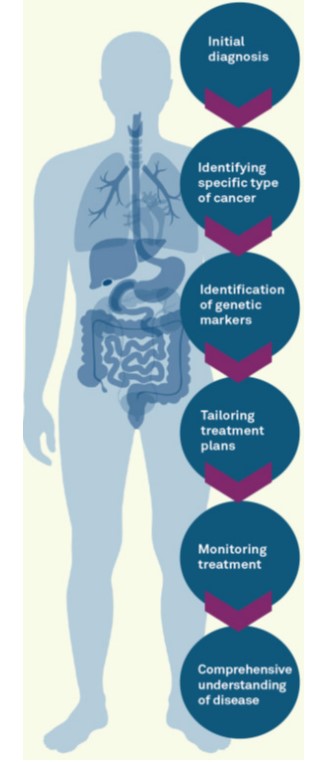 Figure 1: A schematic illustration of the cancer patient journey from
personalized diagnostics to precision therapeutics.
Figure 1: A schematic illustration of the cancer patient journey from
personalized diagnostics to precision therapeutics.However, the responsibility of diagnostic labs extends beyond just ensuring the correct test is ordered. Given the critical nature of time in cancer care, labs are now also focusing on how to accelerate the dissemination of test results to streamline clinical decision-making. OncoDxRx, for instance, is developing precision pathways and collaborating with pathology groups, and their provider organization clients to streamline the process from diagnosis to therapeutic treatment decisions [4,5]. This proactive approach aims to remove gaps in care by offering a comprehensive molecular testing menu, efficient ordering logistics, and actionable results available to the treating physician on day one with the patient.
The authors declare no conflicting interest.
