 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Journal of Medical Research and Surgery (ISSN: 2582-9572) brings articles in the areas related to Medical Research and Surgery on Bimonthly basis.
As opposed to the traditional subscription-based models, open access allows readers to access, use, and share academic work freely and with no charge. This allows for significantly more engagement and provides authors with a stronger platform to share their knowledge and the findings of their research. Since open access grants users’ restrictions-free access to high-quality materials, we have at our disposal a larger array of marketing and promotional tools to expand our readership than traditional publishers typically do. Anyone with a smartphone or a computer and access to the internet will be able to read your manuscript and share it with their peers.
Open access does not mean that authors give up control over their work. In fact, with open access authors are able to maintain the copyright, and readers are required to follow a set of specific rules on how they can correctly use and distribute this work. All work is protected by the Creative Commons Attribution License. The authors' publications in JMRS are distributed under the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/ ). The license was developed to facilitate open access, namely, free immediate access to and unrestricted reuse of original works of all types.
Under this license, authors retain ownership of the copyright for their publications but grant JMRS a non-exclusive license to publish the work in paper form and allow anyone to reuse, distribute and reproduce the content as long as the original work is properly cited.
Appropriate attribution can be provided by simply citing the original work. No permission is required from the authors or the publishers. For any reuse or distribution of a work, users must also make clear the license terms under which the work was published.
The standard license will be applied to the authors' publications, which ensures the publications freely and openly available in perpetuity.
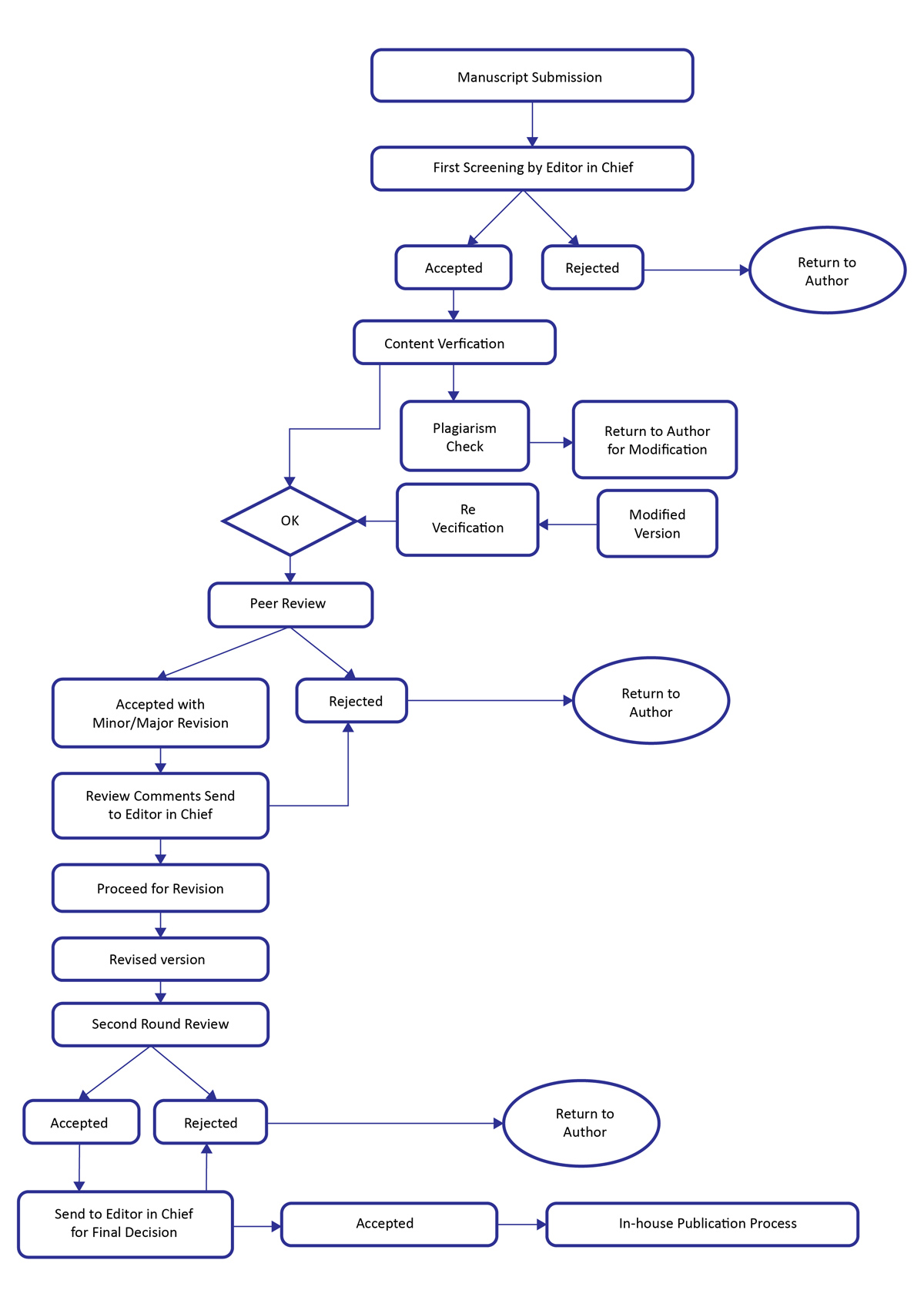
Journal of Medical Research and Surgery (JMRS) follows a double-blind peer review process. An Editor is assigned for the manuscript who checks that the manuscript is appropriate for the journal and is sufficiently original and interesting. After that the manuscript is sent for Preliminary Quality Check. Only after clearing the initial screening, the manuscript is sent to two or more appropriate peer reviewers. If not, it may be rejected without being reviewed any further. As responses are received, further invitations are issued, if necessary, until the required number of acceptances i.e. minimum 2 to 3 is obtained. The reviewers may accept, reject or suggest to revise the manuscript. The reviewers send their comments with a recommendation to accept or reject it – or else with a request for revision (minor or major). The handling editor considers all the reviewers comments before making an overall decision. Based on editor decision whether accept, reject or revise the further steps carried out. If accepted, the manuscript is sent for in house publication process. If the article is rejected, it is sent to authors and in case of major or minor revision, all the reviewers comments are sent to authors to help them to improve the quality of the manuscript. The revised manuscript received from the authors is sent to handling editor for final decision and based on his/her final decision i.e. if accepted sent for in house publication process.
The single most important criterion for acceptance for publication is the originality of the work. Other factors may affect decisions, such factors are, the extent and importance of new information in the paper compared with that in other papers being considered.
The main mechanisms for ensuring the scientific quality of published articles are peer-review, editorial scrutiny. The submitted articles are rigorously peer-reviewed to ensure to accept the high quality submissions, and the quality is controlled by the Editor(s)-in-Chief or editorial board members. The editorial board members and peer reviewers are all experts in their field from various countries and regions around the world. The published articles reflect the up-to-date research findings, with reliable results, objective and un-biased discussion of the results. In the production stage, the copyediting, layout, proofreading, and online publication are all maintained in highest possible quality.
Submission to Journal of Medical Research and Surgery is taken by the journal to mean that all the listed authors have agreed all of the contents, including the author guidelines and author contributions statements. Before submission, the corresponding author ensures that all authors are included in the author list, its order has been agreed by all authors, and that all authors are aware that the paper was submitted. The corresponding author is solely responsible for communicating between the journal and all co-authors, during the manuscript submission, peer review, and publication process.
Authorship needs to be justified by describing the role and contribution of each author on the title page of article as it implies responsibility and accountability for published work.
Authorship roles can vary upon significant intellectual contribution, responsibility and accountability for published work. So, authors sequence and correspondence should be determined by the participants early in the research process, to prevent disputes and misunderstandings. When an author discovers a significant error or inaccuracy in his/her own published work, it is the author's obligation to promptly notify the journal editor or publisher and cooperate with the editor to retract or correct the paper. The journal will not necessarily correct errors after publication if they result from errors that were present on a proof that was not shown to co-authors before publication. The corresponding author is responsible for the accuracy of all content in the proof, in particular that names of co-authors are present and correctly spelled, and that addresses and affiliations are current.
Authors reporting results of original research should present an accurate account of the work performed as well as an objective discussion of its significance. Underlying data should be represented accurately in the manuscript. A paper should contain sufficient detail and references to permit others to replicate the work. Fraudulent or knowingly inaccurate statements constitute unethical behavior and are unacceptable.
The authors should ensure that they have written entirely original works, and if the authors have used the work and/or words of others that this has been appropriately cited or quoted. Multiple, redundant or concurrent publication an author should not in general publish manuscripts describing essentially the same research in more than one journal or primary publication. Parallel submission of the same manuscript to more than one journal constitutes unethical publishing behavior and is unacceptable. Proper acknowledgment of the work of others must always be given. Authors should also cite publications that have been influential in determining the nature of the reported work.
When an author discovers a significant error or inaccuracy in his/her own published work, it is the author’s obligation to promptly notify the journal’s Editor or publisher and cooperate with them to either retract the paper or to publish an appropriate erratum.
Journal of Medical Research and Surgery observes ethics in publication process on the basis of guidelines issued by International Committee of Medical Journal Editors (ICMJE). Journal of Medical Research and Surgery works with the mission of non-discriminatory publication and expects the same from the authors, editors and reviewers. The authors must include an ethics statement describing the details of the committees who approved their experiments. The ethics statement must also confirm that informed consent was obtained from all the recipients. At the time of peer-review, editors may request ethical statement or informed consent letters.
Journal of Medical Research and Surgery expects that authors present accurate, original and objective research in the form of manuscripts. The authors are expected to preserve the raw data and any other valuable information related to the research. The editorial board may review the raw data in relation to the manuscript under publication consideration. The authors are expected to cite properly the publications that have been influential in determining the nature of the reported work. The authors are advised not to publish same manuscripts in more than one journal at the same time. Submitting the same manuscript to more than one Journal or publication will be considered unethical. Such behaviour is convicting and unacceptable. It is the responsibility of the authors to promptly notify editors about the errors and cooperate with them to withdraw or correct the submitted manuscript.
Editors play an important role in scholarly publishing Journal’s. JMRS accept prominent personalities in various research fields, who could carry out their responsibilities with much dedication to improve the quality of the Journal. The editors will be accountable for evaluating the quality quotient of the articles submitted for publication in Journal of Medical Research and Surgery. The editors should ensure that the articles are evaluated according to journal guidelines and constructive feedback is provided to the authors in order to enhance the quality of the articles.
The editors are expected not to disclose any information about a submitted manuscript to anyone other than the corresponding author(s), reviewers and other concerned members of the editorial board.
The editors should ensure that they complete the review process within stipulated time so that manuscripts are processed and reach the publication stage on fast track basis. The consistency in publication will also enforce a feeling of trust among the authors.
Guidelines to be followed
Confidentiality: Reviewers should not share any information from an assigned manuscript with outsiders without the prior permission from the Editor or preserve the data from an assigned manuscript.
Competence: Reviewer with fair expertise should complete the review. Assigned Reviewer with inadequate expertise should feel responsible and may decline the review as it is presumed that reviewer will be an expert in the respective field.
Constructive assessment: Reviewer comments should appreciate positive aspects of the work, identify negative aspects constructively, and indicate the enhancement needed. A reviewer should explain and support his or her judgment clearly enough that Editors and Authors can understand the basis of the comments. The reviewer should ensure that an observation or argument that has been previously reported be accompanied by a relevant citation and should immediately alert the Editor when he or she becomes aware of duplicate publication. A reviewer should not use any kind of abusive language while commenting on an article. Judgment of each article should be done without any bias and personal interest by the assigned reviewer.
Impartiality and Integrity: Reviewer’s decision should solely depend on scientific merit, relevance to the subject, scope of the journal rather than financial, racial, ethnic origin etc., of the authors.
Disclosure of conflict of interest: To the extent feasible, the reviewer should minimize the conflict of interest. In such situation, reviewer should notify the editor describing the conflict of interest.
Timeliness and responsiveness: Reviewers should morally abide to provide the review comments within the stipulated time and be active enough in responding to the queries raised by the editor if any.
The members of the Advisory Board have key policy, regulatory and development responsibilities. Any contribution on behalf of an advisory board member plays an important role in maintaining the integrity of the journal. We therefore cordially invite the Members of the Advisory Council to carry out their work effectively, to help maintain the integrity of the Journal in all respects.
Through Portico, Journal of Medical Research and Surgery offers long-term digital archiving. Worldwide, Portico is a top provider of digital preservation services. The information is kept in an archived form and is not accessible to the general public through Portico, but it is made available when needed in certain situations, such as when the collection is discontinued or the website experiences a catastrophic breakdown.
All authors should disclose in their manuscript any financial or other substantive conflicts of interest that might be construed to influence the results or their interpretation in the manuscript. All sources of financial support for the project should be disclosed.
Any products or services marked as an advertisement or promoted by a sponsor in publications are not endorsed by our Journal of Medical Research and Surgery (JMRS). The content of Editorial is not compromised by commercial or financial interests, or by any specific arrangements with advertising clients or sponsors.
Both types of articles i.e. paid and waivered are not accepted by our Editorial Board Members for publication and printing for advertising purpose. The articles could not linked to advertisings using keywords by authors.
The World Medical Association (WMA) Declaration of Helsinki, as well as good clinical practice guidelines (such as the Good Clinical Practice in Food and Drug Administration (FDA)-Regulated Clinical Trials (USA) or the Medical Research Council Guidelines for Good Clinical Practice in Clinical Trials (UK)), must be followed when conducting research studies involving human or animal subjects. An affirmation that the experiments were carried out with each participant's prior informed consent (written or verbal, if necessary) must be included in any work that describes a study that employed human beings.
When publishing studies involving human subjects, writers should make clear if the practices used complied with the 2008 revision of the 1975 Helsinki Declaration as well as the institutional and national accountable committee on human experimentation's ethical requirements. If there is any uncertainty about whether the research was carried out in line with the Helsinki Declaration, the author(s) must defend their method and show that the institutional review board specifically authorized the study's questionable components.
Authors should note if institutional and national guidelines for the care and use of laboratory animals were followed when reporting on experiments involving animals. Vertebrate or other regulated invertebrate experimental research must adhere to institutional, governmental, or international regulations and, when possible, should have received the appropriate ethical committee's approval. The work must have a statement describing adherence to rules and/or ethical approval. The author(s) of research involving client-owned animals must show that the client gave informed consent and that the study was conducted in accordance with best practices in veterinary medicine.
The Editor-In-Chief will evaluate the submitted manuscripts on animal well-being issues and if the research found to be inconsistent with commonly accepted norms of using the animal and human subjects, the manuscript is liable to be rejected. The Editor-In-Chief also reserve the right to contact the approving committee for any further clarification. Researchers please note that they have a moral obligation towards the animals and human subjects they use for their research goals and they must treat them with compassion and consider their well-being while designing the projects.
 Plagiarism, duplicate submission, data falsification, inappropriate authorship credit, and all the like misconducts are not tolerated. This journal uses PlagScan Software to detect any possible plagiarism in the submissions.
Plagiarism, duplicate submission, data falsification, inappropriate authorship credit, and all the like misconducts are not tolerated. This journal uses PlagScan Software to detect any possible plagiarism in the submissions.
Informed consent is required for any paper to be published in Journal of Medical Research and Surgery if that research involves human participants. Informed Consent policy states that a participant in research must be informed about all aspects of the trial and the research should be carried out only when the participant voluntarily confirms his or her willingness to participate in a particular clinical trial and significance of the research for advancement of medical knowledge and social welfare.
In case report(s), the authors should include a statement that the patient's consent has been obtained if the patient's identifying information, such as name, hospital number, and any other privacy nature items will be published due to the information are essential for the scientific purposes. In the manuscript, there should be such statement "Patient's informed consent for publication of this report was obtained". If there is not any identifying information in the manuscript, authors may state "Informed Consent: Not Applicable".
In most clinical studies (trials), retrospective or prospective nature, with human subjects involved, the individual patient informed consent is required; there should be such statement in the manuscript. If such informed consent is not necessary or exempted judged by the Ethical Committee (such as in some retrospective studies), the authors should clearly state such waiver of informed consent in the manuscript.
If the patient is deceased, the authors should seek informed consent from a relative of the patient. If neither the patient nor a relative can provide such consent, the manuscript can only be accepted and published if the identifying information has been sufficiently anonymized and covered.
In particular, please remove any identifying information (patient name, birth, hospital number, etc) in the images, ECG report, pathology slides, X-ray, ultrasound and any other imaging data prior to submission, if these information are not relevant to scientific purposes.
The authors should keep the signed informed consent form; a copy should be available upon journal editor's request.
Journal of Medical Research and Surgery completely follows the regulations from ICMJE. Also, Journal of Medical Research and Surgery is a member of ICMJE at present.
Prior to final publication: The manuscript is thoroughly checked by the peer-review council after the author sends the revised manuscript, to ensure that recommended suggestions are implemented in the article content. Thereafter, Galley proofs are sent to the author for proofreading and corrections are done before the PDF's are sent for final publication. After final publication: Once the article is finally published, changes with regard to author names, affiliations etc. will not be entertained. However, if any mistake is detected that seriously affects the publication records or the scope of the article then modifications can be done on receipt of such request from the corresponding author of the article.
Policies related to retraction of the articles covers instances leading to unethical practices in developing an article, multiple submission, fake authorship, and any other fraudulent conduct. It also covers requests to retract an article for correcting mistakes and technical errors in the research data. We adopt to the best practices for dealing with retractions and allow genuine requests on receiving such requests with a retraction request letter duly signed by the signed by the authors. After approval, the HTML and other versions of the article are removed from the Journal of Medical Research and Surgery database and other promotional platforms.
Manuscripts may be withdrawn by submitting a letter to the editorial office stating the reasons for manuscript withdrawal. There are no withdrawal charges if the manuscript is withdrawn before plagiarism check but the authors need to pay 50% of the publication fee as Withdrawal charges after 7 days of the manuscript submission.
Replacement of the article is accepted in cases where the article, if acted upon, might pose a serious health risk and the authors of the original article wish to retract the original and replace it with a corrected version.
The above policies are designed and implemented to address any concern of the authors and to take into account best practice in the scholarly publishing. These policies are revisited on scheduled basis and revised as per the international standards and best practices adopted by the publishing and information industry.
Research misconduct means fabricating, falsifying, manipulating citations or plagiarism during the production, performance or evaluation of research and writing by the authors or in the process of communicate research results. When authors are found to be involved in research or other serious misconduct in peer-reviewed journal articles, the editors are responsible for ensuring the accuracy and integrity of the scientific records. Claims or allegations of misconduct will be taken seriously, whether the individuals involved are internal or external to the journal, or whether the submission in question is before or after publication.
Process for making a complaint: Complaints must be sent by e-mail to , so that they can be verified as best as possible. Complaints will be confirmed within 3 business days of receipt and complainants will be notified of the process and expected timelines from that point until a resolution is found. If the complainant is not satisfied with the response, they may request that the complaint be referred to the Editorial Lead, who will review and monitor the matter from that point on. If the matter involves allegations against a member of the publisher's team, senior management will be notified to review and monitor the investigation. If a conflict of interest becomes apparent, one or more independent and objective persons may be sought to conduct the investigation. Process for handling the complaints: The first step is to determine the validity of the allegation and assess whether the allegation meets the definition of research misconduct. This first step also involves determining if the alleged wrongdoers have a relevant conflict of interest. If there is a possibility of scientific misconduct or the presence of other significant research anomalies, the allegations will be shared with the respective author, who, on behalf of all co-authors, is requested to ask for detailed feedback. Once feedback has been received and evaluated, further evaluation and input from experts (such as statistical reviewers) can be obtained. In the unlikely event that an error has occurred, clarification, additional analysis, or both, is published as a letter to the editor, and usually includes a notice of correction and correction of the published articles is enough. Organizations should conduct an appropriate and thorough investigation into allegations of scientific misconduct. Finally, authors, journals and institutions have an important obligation to ensure the accuracy of scientific records. By responding appropriately to concerns about scientific misconduct and taking necessary action based on an assessment of those concerns, such as corrections, withdrawals with replacements, and withdrawals, The JMRS journal will continue to be responsible for ensuring the validity and integrity of the scientific record.
Appeals against editorial decisions will only be considered under very specific circumstances, and usually only when a clear policy violation can be demonstrated or the author can point out a clear misunderstanding about the reviewer's paper.
Rejected manuscripts: The most common reasons for rejecting manuscripts are: The content of the article is beyond the scope of review; Articles are not written in clear, Understandable English; The article does not meet our guidelines for authors for content, style, and/or format; The article does not meet the journal quality as recommended by the reviewer and the editor's decision. In the last three cases, articles are usually reopened for author editing within 8 weeks. Failure to meet this deadline will result in the manuscript being automatically rejected. We will not consider appeals against publisher decisions in any of these cases. It is the author's responsibility to provide accurate contact details, follow up mail from our office, respond promptly with the correct email address, and comply with our requests. When a manuscript has been rejected because the authors did not honor the review deadline, resubmission is possible.
Rejection of revised articles: Revised articles will generally not be rejected as long as they follow our guidelines for revised versions. We will not consider appeals to the editorial board's decision to reject an amended article if it does not meet our requirements. Authors whose manuscripts have been rejected for other reasons may follow the appeals process if they wish to appeal, but note that editors are unlikely to be able to reverse their original decision unless there is an appeal. Important new information is provided or may indicate that there is an error in our process.
Retracted articles: Editors do not make the decision to remove articles lightly and will often conduct a thorough investigation before doing so. We will only consider withdrawal appeals if we can provide substantial evidence that the decision was unfair.
