 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Emma Dioso1, John Cerillo2, Mohammed Azab3, Devon Foster4, Isaac Smith4, Owen Leary5, Michael Goutnik4, Brandon LuckeWold4*
1Department of Neurosurgery, University of Utah, Salt Lake City, UT
2Nova Southeastern University, Dr. Kiran C. Patel College of Osteopathic Medicine, Clearwater, FL
3University of Boise State, Biomedical Science Department, Boise, ID
4University of Florida, Department of Neurosurgery, Gainesville, FL
5Brown University, Department of Neurosurgery, Providence, RI
Correspondence to: Brandon Lucke-Wold, University of Florida, Department of Neurosurgery, Gainesville, FL
Received date: June 23, 2022; Accepted date: July 13, 2022; Published date: July 20, 2022
Citation: Dioso E, Cerillo J, Azab M, et al. Subconcussion, Concussion, and Cognitive Decline: The Impact of Sports Related Collisions. J Med Res Surg. 2022; 3(4):
54-63. doi: 10.52916/jmrs224081
Copyright: ©2022 Dioso E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Subconcussion can cause long-term consequences for patients. Increasing understanding of what causes the injury and how it can be assessed is important. This paper focuses on the pathophysiology, epidemiology, and assessment tools. Specific emphasis is placed on early diagnosis to implement treatment. Current research is targeting improved pharmaceutic and biomechanic innovations. Enhanced understanding of subconcussion will improve outcomes for patients and allow clinicians to implement treatments earlier.
Subconcussion, Assessment, Interventions, Future discoveries.
While subconcussive injuries are not novel in anecdotal observations or practice, as Martland vividly used the term “punch drunk” in 1928, the literature surrounding the definition of subconcussion in emerging. Subconcussive head injuries are defined as injuries in which an impact to the head does not result in clinical symptoms of a concussion. That is, while the injury is the result of a traumatic impact from a biomechanical force to the head and/or upper body, the impact is not enough to reach the threshold needed to produce concussive symptoms [1]. Contact and noncontact sports give rise to many commonplace scenarios that could potentially give rise to subconcussions, taking a charge on a basketball court, hitting the wall backstroking during a swim meet, heading a ball in soccer, and various others. While each impact may not cause enough damage to reach threshold of noticeable symptoms, repetition of these subconcussive injuries may compound over time [1]. Together, these cumulative injuries do have dramatic impacts due to the microstructural and functional changes that result in the brain [1,2].
Chronic Traumatic Encephalopathy (CTE) is the neurodegenerative syndrome associated with repetitive subconcussive traumatic injuries [3]. CTE is characterized by the “accumulation of abnormal tau in neurons and astroglia distributed around small blood vessels at the depths of the sulci”[4]. Notably, long-term consequences of subconcussive injuries more commonly present as dementia without p-tau pathology or Alzheimer’s disease-like-β-amyloid deposits [5,6].
Patients with histories of subconcussive injuries and other brain trauma that develop dementia often characteristically exhibit deficits in their episodic, working and spatial memory [7-9]. As a single subconcussive injury by definition causes no apparent clinical symptoms, the mechanism by which cumulative subconcussive injuries lead to such detrimental outcomes is not well understood, especially as time between events is seemingly not significant. Consequently, the delayed onset of debilitating symptoms and memory loss deem the progression of subconcussive injuries challenging to evaluate in human and mouse models, though new data and possible mechanisms are emerging [10].
Former athletes developing aggressive behaviors, loss of control, impaired attention, depression, memory loss, and executive dysfunction associated with CTE have been described in the literature on numerous occasions [11]. In 2013, Stern and colleagues described two clinical presentations of CTE. The first variant presents with mood and behavior changes at an average of 35 and progresses to severe cognitive symptoms. The second variant presents with cognitive symptoms at an average of 60 and progresses to encompass mood and behavioral changes [12]. As each impact may cause mechanical loading of sensory and neural components within the head, various structures contributing to sensorimotor control of balance may also be impacted [13].
Concussions and subconcussions are a result of rapid acceleration or deceleration in a linear or rotational plane [14- 16]. Most commonly these fast-paced injuries occur during falls, Motor Vehicle Accidents (MVAs), or Sports Related Collisions (SRCs) [15,17]. A direct or indirect blow to the head and neck can cause the brain and skull to “shake together violently” as intended through the latin root concussus. These injuries lead to a disruption in the neuronal membrane causing an efflux of potassium, influx of calcium, and increased glutamate concentrations [18-20]. A 2015 study on colligate football players using serial fMRI 1 day, 1 week, and 1 month postconcussion showed that return of cerebral blood flow was positively correlated with player recovery [21]. Furthermore, decreased cerebral blood flow along with increased lactic acid production and intracellular Ca2+ leads to a continuation of cellular damage [18-20]. These neurometabolic changes lead to chronic depolarization, cellular damage and eventually cognitive suppression [16,18-20]. Literature suggests when these head injuries occur in children and adolescents, long term cognitive and behavioral deficits may persist [22,23].
From an SRC standpoint, head impacts potentially leading to concussion and sub-concussion can occur during practice, gameplay, and celebration [24-26]. A collegiate ice hockey study from 2014 showed that only half of recorded head impacts were caused between players while the other half occurred with the environment (boards, ice) or equipment; therefore, it is important to consider non player-to-player contact [25]. It is consistently noted throughout literature, however, that the vast majority of sports concussions occur due to collision between players [26,27].
A study from the University of Florida collected practice drill impact numbers during the 2016-2017 & 2017-2018 football seasons and found that an average lineman experienced a head impact >10 g once every 8.3 minutes of drill time. Asken et.al found that limiting time of specific drill types could lead to decreased exposure of repetitive head impacts [24].
Adolescent, colligate, and professional sports have been the focus of concussion research for the past decade. Studying these populations is vital, as contact sports including football, soccer, hockey, wrestling, boxing, lacrosse, and basketball have shown to consistently serve as the most prevalent producers of head injuries and subsequent concussions (Figure 1 and Figure 2) [19,26,28-31].
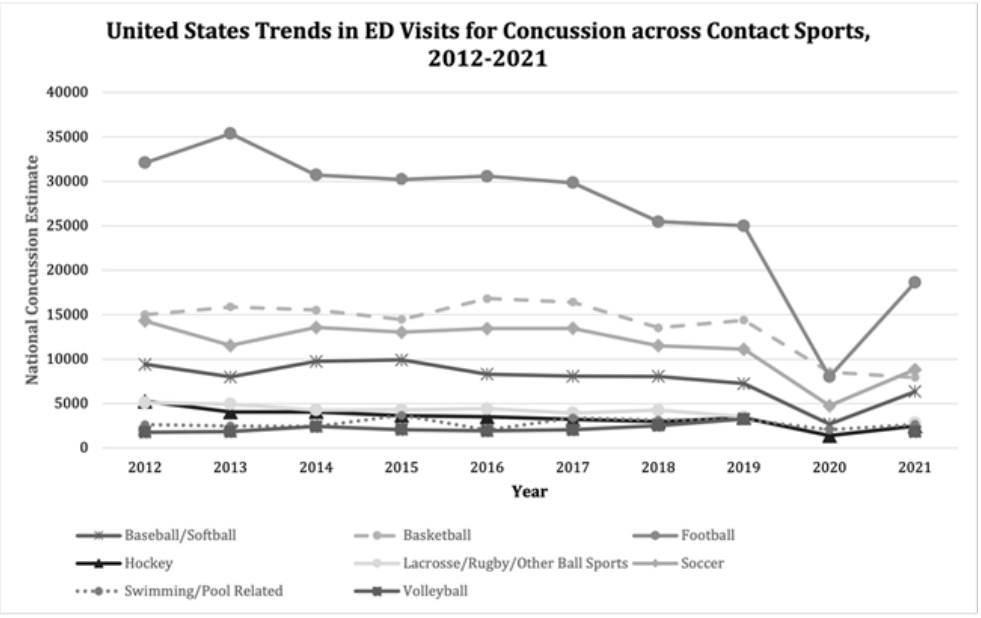 Figure 1: National trends in number of emergency department (ED) visits for
concussion due to various contact and non-contact sports among persons of
all ages, as estimated by the National Electronic Injury Surveillance System
(NEISS), United States, 2012-2021. Annual gaps in NEISS estimates are due to
estimate of less than 1,200 cases, less than 20 cases available for estimate, or
coefficient of variation exceeding 33%.
Figure 1: National trends in number of emergency department (ED) visits for
concussion due to various contact and non-contact sports among persons of
all ages, as estimated by the National Electronic Injury Surveillance System
(NEISS), United States, 2012-2021. Annual gaps in NEISS estimates are due to
estimate of less than 1,200 cases, less than 20 cases available for estimate, or
coefficient of variation exceeding 33%.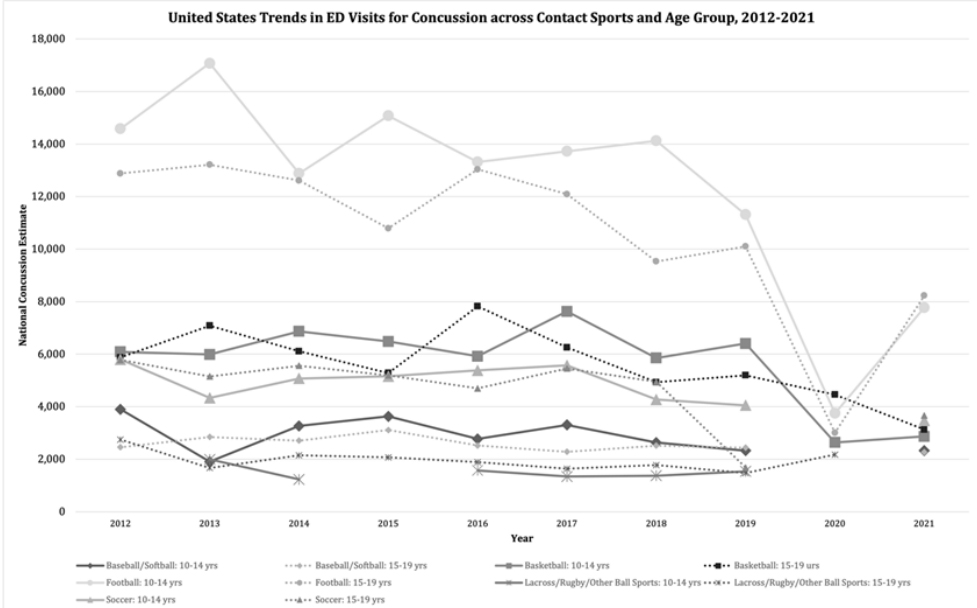 Figure 2: National trends in number of emergency department (ED) visits for
concussion due to contact sports and non-contact sports among athlete age
group, as estimated by the NEISS, United States, 2012-2021. Annual gaps in
NEISS estimates are due to estimate of less than 1,200 cases, less than 20
cases available for estimate, or coefficient of variation exceeding 33%.
Figure 2: National trends in number of emergency department (ED) visits for
concussion due to contact sports and non-contact sports among athlete age
group, as estimated by the NEISS, United States, 2012-2021. Annual gaps in
NEISS estimates are due to estimate of less than 1,200 cases, less than 20
cases available for estimate, or coefficient of variation exceeding 33%.Veliz et al., reported that 28% of collegiate athletes between 2014-2016 reported at-least one concussion over their lifetime [32]. In an additional study, Veliz et al., showed that the number of students (grades 8,10,12) who self-reported at-least one concussion grew from nearly 20% to 24% between 2016 and 2020, however this survey was not focused on student athletes [33]. Although these number are growing, Lincoln et.al described the increase incidence of concussions in youth sports be due to increased reporting rather than actual increased occurances [28]. In contrast to the prior studies, the National Electronic Injury Surveillance System (NEISS-AIP) found that concussion rates among <17-year-old athletes declined 27% between 2001-2018. The NEISS-AIP system recorded data from a representative sample of U.S emergency departments and found that 45% of head injury visits among adolescents were due to contact sports [34,35].
As for professional sports, A 2018 study on concussion numbers in National Football League (NFL) reported an average greater than one concussion for every two games [36]. While a 2022 study reported concussion prevalence in the National Hockey League (NHL) to be one concussion for every 17 games [37].
Traumatic brain injuries are associated with significant morbidities and mortalities [17]. According to CDC surveillance study, TBIs related deaths represented about 30% of all injury related deaths [38]. Moreover, the rate of emergency room visits due to all TBIs increased by 70% from 2001 to 2010 [39]. The increased rate of TBI-related hospitalization may be related to the increased awareness among people regarding the hazards of concussion and related consequences leading to more ER visits [17]. TBIs related health services are considered highly costly for the community [40]. The national institute of health reported that the costs of TBIs exceeds other chronic medical conditions [41]. The rates of admission are higher among the elderly people (age >75), (2,232.2 per 100,000 population) and those 15-24 years (1,080.7) according to a CDC report [17]. In recent years, studies have reported a marked increase in the number of annual pediatric ED visits related to concussion [42]. About 5.3 million Americans are suffering from TBI related disabilities [43]. The rates of hospitalizations and deaths were also higher among the elderly group [17].
Studies involving the medical costs of TBIs are deficient [44]. The study conducted by Brooks and colleagues involved only a small sample of population [45]. While McGarry and colleagues investigated a larger population, they excluded the young folks [45,46]. Moreover, the assessment of the costs included only those covered by health maintenance organizations and those associated with acute care services [47,48]. The early treatment of intracranial complications of TBIs and the somatic, affective, and cognitive complications are expensive. The highest individual costs are caused by the most severe forms of TBI [49]. Research is directed towards implementing methods to study the costs of health care services related to TBIs and to mitigate the costs.
Health care utilization and the possible related aspects are poorly characterized especially in persons living with TBI sequelae [50]. The systems of care for TBIs vary between and within countries [51]. The clinical care setting is variable and ranges from immediate on-site management to long term care. This variation in care is due to the heterogeneity in resources and financial settings [52]. The emergency room is the initial meeting zone with the concussion patient. Transfer of the patients from non-specialist hospitals to highly specialized trauma centers have a positive impact on outcome and costs [53,54]. Several studies categorized TBI patients regarding the utilization of health services into superutilizers and underutilizers [55,56]. Salisbury and colleagues considered superutilizers as those patients who have more than 3 hospital encounters per year one year after trauma which was the same for the study conducted by Eliacin and colleagues [56]. Between 2001 and 2009, the number of children seeking treatment for mild TBI increased by 62%. [57]. A large proportion of pediatric population presenting with concussion undergo CT scan for diagnosis [58,59]. A variety of studies have been designed to categorize the patients with TBIs according to the need for CT scan in the ED [60-62]. In the United States, about one-third of the 80 million CTs requested each year are ordered from the ED, nearly half of them involve the brain [63]. There is not much evidence on the methods of pain management and their related costs for patients presenting with concussion [64]. Meticulous research should be designed to develop ways to reduce the unnecessary admissions and resource utilization for superutilizers. Among TBI survivors, there is not much data regarding the aspects of health care utilization long-term (Figure 3) [56].
Signs and symptoms of concussion may potentially be delayed, subtle, or variable in nature. Reliance on athlete-reported symptoms make diagnosing concussions on the spot a challenge when determining if it is safe for an athlete to resume activity [65]. Current assessment strategies for assessing concussions start at recognition of injury. After identifying an injury with the potential of causing a concussion, an assessment of symptoms is performed where cognitive function, cranial nerve function, balance, and potential intercranial bleeding are assessed [66- 68].
While concussions exhibit symptoms that can be observed, subconcussions do not, making them more difficult to diagnose. This makes it difficult to determine if it is appropriate for an athlete to return to activity. Acute repetitive trauma to the head has been linked to long-term risk for cognitive decline, neurobehavioral changes, and neurodegenerative disease. Due to the difficulty in diagnosing subconcussions due to their lack of symptoms, removing athletes from a sporting event after experiencing a potentially traumatic head impact is used as a preventative measure [69-71].
The Immediate Postconcussion Assessment and Cognitive Testing 2.0 program (ImPACT) is a computerized test used to manage sports related concussions by testing cognitive function of athletes before and after a concussion [72]. Measurements collected by head-impact monitoring systems provided realtime data, but lacked clinical utility due to error rates and low specificity in predictive concussion [73] However, these monitoring systems did indicate that sustaining an impact of 90 g does not result in acute observable balance and neurocognitive impairments [74]. This monitoring system is unique in that it offers to provide objective data, not of the results of an impact of a head injury, but the impact itself -something that the ImPACT assessment is incapable of. An Electroencephalographic (EEG) index has been used to track quantitative brain activity in a group of athletes recovering from concussion. An algorithm was developed for normal brain electrical activity and compared to the abnormal features of brain electrical activity that was detected in athletes recovering from concussion [75]. While similar to that of the ImPACT assessment in that measurements can be compared from before and after a concussion occurred, quantitative EEG does not appear useful as a routine screening measure of traumatic brain injury or postconcussive symptoms (Figure 4) [76].
Concussive effects on learning are well reported: increased irritability and frustration, decreased attention, difficulty with memory [77,78]. Subconcussive pathology, despite the disease being poorly understood overall, is also well reported, with CTE being perhaps the most notable long-term sequala [79-81]. A positive correlation exists between the frequency and magnitude of subconcussive head injury and levels of TBI biomarkers in the blood [82]. Variability in default mode network signaling has also been found in brains of high school football players in a manner consistent with the effects of concussive blows [83].
Subconcussive injury’s relationship to learning, however, is not well understood. While sharing similar pathophysiology with its more severe counterpart, the absence of clinical signs is a hallmark of subconcussive TBI. This makes recognizing patterns in the classroom elusive as no valid academic metrics identifying subconcussive TBI exist.
Talavage et al. [84], found that significantly lower ImPACT neurocognitive test scores corresponded with dorsolateral prefrontal cortex injury in football players during the season. Another study compared ImPACT scores between nonconcussed athletes of high-and low-contact sports and found significant differences in Processing Speed and Reaction Time scores, but not in Verbal Memory, Visual Memory, or Total Symptoms [85]. Several studies have examined the effects of headers in soccer on school-age children and failed to establish a correlation between repeated exposure to the subconcussive hits and diminished neuropsychological performance [86]. It is important to note, therefore, that across these studies and in the literature more broadly, subconcussed patients are asymptomatic. In the literature, the deficits are identified only because the students are enrolled in TBI studies. Of the subconcussion group, Talavage et al. [84], writes that the “players failed to accrue sufficient short-term damage to integrative neural systems that they exhibited externally observable symptoms”. This is emblematic of how the disease manifests in the classroom.
Bearing in mind that repeated subconcussive injury predisposes to concussion and has significant, symptomatic long-term effects, the absence of cardinal academic signs should not lead one to conclude the relationship is benign [80,87]. It may be that the brain is too resilient for subconcussive injury to effect learning in the timeframes studied, or that the severity and rate of manifestation depends on duration and magnitude of subconcussive exposure. As Ntikas et al. [88], suggested, knowing more about the disease does not ensure that a reliable gold-standard assessment, relying on gross cognitive deficits, would pick up on the effects of subconcussive trauma. Ultimately, the effects on learning will be greatly aided by a more precise definition of what constitutes subconcussive TBI and by enhanced understanding of the mechanism of disease (Table 1) [81,88].
Study |
Inclusion |
Outcomes |
Findings |
| Functionally-Detected Cognitive Impairment in High School Football Players without ClinicallyDiagnosed Concussion [84] | 23 males, ages 15-19 years. High school football. | Head collisions during season measured with HIT, ImPACT neurocognitive testing before, during, and after season. fMRI imaging. | 4 participants exhibited reduced ImPACT scores, reduced fMRI activity in DLPFC despite no clinical signs of TBI. |
| Are There Subconcussive Neuropsychological Effects in Youth Sports? An Exploratory Study of High- and Low-Contact Sports [85] | 282 nonconcussed male high school athletes. High- and low-contact sports. | ImPACT scores | Significantly faster ImPACT Processing Speed and Reaction Time in low-contact group. |
| Effects of sub-concussion on neuropsychological performance and its potential mechanisms: A narrative review [86] | 18 studies relevant to learning reviewed. | Neuropsych. impairment, neuromotor deficit, memory, attention. | Evidence in the literature conflicts on neuropsych, impairment, neuromotor deficits, and memory impairment. Subconcussion does impair attention. |
Concussive and subconcussive brain injuries can lead to occupational challenges for affected individuals across the age spectrum, as well as problems with educational activities, sleep, social participation, and activities of daily living [89-91]. Such challenges following mild TBI are common and impose a significant economic burden on the healthcare system [92]. Among secondary school- and college-aged students experiencing concussive injuries, a population in which mild TBI is particularly prevalent, ongoing occupational performance limitations have been reported in as many as 89% of affected patients [91]. Further, it has been observed that children with mild TBI may be even more likely to have unmet functional needs related to education and occupational limitations compared to patients with moderate and severe injuries, in whom limitations may be more readily apparent [93]. Accordingly, proactive implementation of occupational therapy interventions may improve time to neurologic recovery and promote rapid return to function [89,94].
Several observational and pilot studies have been conducted to investigate the impact of occupational therapy and various related interventions on functional improvement following TBI, though there remains significant opportunity for further study of such interventions in mild TBI specifically. First of all, including occupational therapists in the initial evaluation of patients with mild TBI has been shown to improve identification of functional impairments that may otherwise have been missed [95]. Early focus on improving executive function during acute inpatient rehabilitation following TBI has been shown to modestly improve community participation and functional independence 1 year after discharge in a heterogenous cohort of patients with complicated mild, moderate, and severe TBI [96]. Similarly, increasing the proportion of contextualized functional therapies (versus decontextualized, clinic-based therapies) as part of the OT regimen following TBI has been found to increase treatment efficacy in a similarly heterogeneous cohort [97]. Oculomotor impairment has been implicated as a contributor to long-term functional impairment following mild TBI, and pilot study data has suggested that closer collaboration between occupational therapy and optometry may be indicated for patients with these symptoms [98]. Occupational therapy targeting impairments in ability to drive a vehicle have also been shown to be effective in a cohort of returning combat veterans who suffered mild TBI as well as other injuries [99]. Finally, there is some early evidence that better patient engagement with occupational therapy after sustaining combat-related mild TBI may somewhat mitigate risk of suicidal ideation [100].
Challenges persist in the identification of effective occupational therapies for patients with mild TBI, as well as in maximizing the utilization of available resources. The intrinsic heterogeneity of TBI makes it difficult to study, an issue further exacerbated by the limited availability of high-quality evaluation tools particularly for those with mild injuries [96,101]. Furthermore, there are a lack of evidence-based guidelines related to when occupational therapy should be employed in the post-TBI recovery process, leading to inconsistent referral patterns, and which techniques should be utilized, though practice recommendations have been previously published amidst these limitations (Clinical Practice Guidance: Occupational Therapy and Physical Therapy for Mild Traumatic Brain Injury, 2009) [95,102-105]. Recent studies, including a survey of clinicians caring for pediatric mild TBI patients, have identified that occupational therapy is routinely utilized for only a minority of patients who may benefit from it following mild TBI [103,105]. Another study contextualized this trend by identifying that utilization may be disparate with respect to geographic and/or socioeconomic factors [106]. Another study which surveyed practicing occupational therapists regarding their confidence in managing concussion and mild TBI found clinician comfort to be highly variable, with clinicians who proactively engaged in continuing education related to concussion being more confident in managing patients with these injuries [107].
There is a great need for research into imaging, molecular, and clinical correlates of subconcussive and concussive events. Using advanced neuroimaging, including modalities like Transcranial Magnetic Stimulation (TMS) and Diffusion Tensor Imaging (DTI), to identify those at risk of long-term sequelae of repetitive subconcussive hits is an area to explore [70,108-110]. For example, using temporal and spatial data from helmets during a football season, combined with fractional anisotropy (measured via DTI), may help inform return to play decisions [108]. Similarly, TMS may help confirm corticomotor inhibition and GABAergic dysfunction after repetitive subconcussive injury [109,110]. Future work should apply TMS on a larger scale and perhaps incorporate dose-response.
Some biomarkers like Neurofilament Light (NfL), a biomarker of axonal injury, may potentially be tracked over a full season with thresholds set for predicting those with potential for long term consequences [70]. Furthermore, as demonstrated in a rat model, repetitive subconcussive injury may lead to neuromotor decline as a result of ventriculomegaly, which itself results from a perturbation of the blood-brain barrier [111]. Hiles-Murison et al., hypothesized the increased permeability of the bloodbrain barrier may result from increased systemic inflammation [111], but did not find any signs of neuroinflammation, which may be the result of only two weeks of subconcussive injury exposure. The authors did find non-significant lipid olefinic reductions in the corpus callosum and cortex of rats exposed to repetitive subconcussive impacts [111]. Thus, more work needs to be done to assess the potential role of inflammation and lipid homeostasis in the pathogenesis of repetitive subconcussive injury, as inflammatory and lipid biomarkers may perhaps be able to assess the “dose” of injury and predict long-term effects. Other work has highlighted the potential role of salivary IgA autoantibodies to the brain-enriched and apoptosis-regulating proteins HTR1A, SRRM4, and FAS [112]. This is promising in that obtaining saliva is non-invasive and may provide rapid evaluation.
Clinical measures and cognitive assessments may also help quickly assess the effects of cumulative impacts. For example, ocular motor examination may serve useful in diagnosing patients and predicting effects of subconcussive and concussive impacts [113]. Furthermore, Electroencephalography (EEG) has been successfully employed to assess the dose response of repetitive subconcussive injury on brain vital signs [114]. Thus, with enough easily obtained data, more specific cognitive assessments may be developed in routine practice.
Understanding the mechanism of injury and the tolerance within humans in regard to repetitive subconcussive injuries is vital in developing diagnosis and treatment models. A relationship between repetitive subconcussive injuries and the resulting insidious behavioral and pathological outcomes has yet to be defined in a study that translates to human physiology and subconcussive biomechanical characteristics [115-118]. Much of the human studies literature is subject to significant variability, particularly in frequency and magnitude of subconcussive impacts. Preclinical models to define a causative relationship of impact to outcome are promising, as they provide the most control of variables. In 2022, Stemper and colleagues developed a preliminary preclinical model incorporating head rotational acceleration. Their data suggests the ability to scale human subconcussive injury characteristics down to a rodent model with greater accuracy in order to assess the behavioral and physical consequences [119]. Further research in understanding the mechanism and thresholds of subconcussive injury specific to humans is necessary and ongoing, particularly in regard to frequency of injury exposures and resulting long-term symptoms.
Acute sensorimotor changes (i.e., standing balance) have also been detected in athletes that experienced a head impact that did not result clinical concussions, suggesting subconcussive injuries [13,120,121]. Balance assessments are commonplace within clinical concussion evaluations, as 30% of patients with concussions from sports related injuries exhibit acute balance problems [122]. 67-77% of patients report dizziness [26] and 69% exhibit visual deficits [123], likely also contributing to balance problems. While direct head impacts are believed to acutely affect sensorimotor function via mechanical deformations of brain tissue [124], much of literature focuses on the biomechanical parameters regarding concussions. A considerable gap exists in understanding the biomechanical factors within subconcussive injuries and the resulting acute sensorimotor outcomes. In 2021, Qiao and colleagues sought to evaluate a link between head impact biomechanics and resulting balance deficits. They found substantial variability and inconsistencies among balance testing studies following subconcussive injuries [13]. Further research with consideration for the subtly and variety of these sensorimotor deficits in subconcussive injuries, compared to the grandiosity of the deficits in concussive injuries, is necessary.
At a cellular level, the relationship between multiple serum biomarkers and repeated subconcussive injuries has become an area of study. Multiple groups have studied increased serum NfL as a measure of repeated subconcussive injuries in soccer and American football athletes [125-129]. Other studies have evaluated S100B levels before and after football practice, suggesting increased S100B with increased frequency of subconcussive impacts acutely, but not over the entire season [130]. Other studies found GFAP, ubiquitin C-terminal hydrolase-L1 (UCH-L1), and S100B biomarkers to all show an increase in patients with subconcussive injuries versus controls [131]. In a 2021 review of the literature, Hier and colleagues found GFAP, S100B and Glial Fibrillary Acidic Protein (GFAP) exhibited a modest ability to discriminate between CTpositive and CT-negative subconcussive patients, though more accurately than tau and NfL. Literature regarding the use of these biomarkers to predict severity of injury and outcomes is variable and inconclusive [132]. Additionally, literature regarding alternate routes of biomarker entry to blood in growing, suggesting a disruption of the blood brain barrier is not necessary for entry (i.e., through intramural periarterial drainage system and glymphatic system) [133]. Preliminary research regarding the use of serum biomarkers in the diagnosis and treatment of subconcussive injuries is a promising concept, but requires much further evaluation into the nuances and confounding factors.
The authors have no known conflict of interest to disclose.
No


