 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Gerardo Alberto Solis Pérez1* , Tomás Segura Fernández2
, Tomás Segura Fernández2 , Diego Escarraman Martinez3
, Diego Escarraman Martinez3 , Luz Elena Carpio Dominguez4, Manuel Alberto Guerrero Gutierrez5
, Luz Elena Carpio Dominguez4, Manuel Alberto Guerrero Gutierrez5 , Joana Elizeth Hernández Hernández4, Jorge Mario Antolinez Motta6
, Joana Elizeth Hernández Hernández4, Jorge Mario Antolinez Motta6 , Arturo Vázquez Peralta7
, Arturo Vázquez Peralta7 , Raymundo Flores Ramírez8
, Raymundo Flores Ramírez8 , Elvia Darelly Reyez Pérez9
, Elvia Darelly Reyez Pérez9
1Thoracic Anesthesiologist, Unidad Medica de Alta especialidad número 14, Adolfo Ruiz Cortines, IMSS Veracruz, Veracurz, México.
2Thoracic Anesthesiologist, Instituto Nacional de Enfermedades Respiratorias, Ciudad de México, México.
3Anesthesiologist, UMAE Hospital de Especialidades “La Raza”, IMSS, Mexico City, Mexico.
4Thoracic Anesthesiologist, National Institute of Respiratory Diseases Ismael Cosío Villegas, México.
5Anesthesiologist, Intensive Therapy, Department of Bariatric Anesthesiology at the Autonomous University of Baja California, México.
6High Perioperative Risk Clinic, Hospital General Dr Manuel Gea González, Ciudad de México, México.
7Cardiovascular Anesthesiologist, Hospital Central Sur de Alta Especialidad Pemex, México.
8Department of Internal Medicine, Hospital, ISSSTEP Puebla, México.
9Hospital General de zona Número 71, Lic. Benito Coquet Lagunes, IMSS, Veracruz, México.
Correspondence to: Tomás Segura Fernández, Thoracic Anesthesiologist, Instituto Nacional de Enfermedades Respiratorias, Ciudad de México, México.
Received date: June 28, 2023; Accepted date: July 05, 2023; Published date: July 12, 2023
Citation: Solis PGA, Segura FT, Escarraman MD, et al. Spirometry: A Test Underestimated by the Anesthesiologist J Med Res Surg. 2023;4(3):63-69. doi: 10.52916/jmrs234110
Copyright: ©2023 Solis PGA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Spirometry is a lung function test, whose main objective is to evaluate lung mechanics. It is a test that is currently easily accessible, but little used by anesthesiologists. One cause may be that they are not familiar with the analysis and understanding of said test or the lack of a pulmonary physiology laboratory in their hospitals. The proper analysis and understanding of this test by the general and thoracic anesthetist offers a range of possibilities both for the perioperative evaluation of the patient with pulmonary pathology or the one who will undergo thoracic surgery. Having a great impact by influencing the prognosis and management of these patients.
Spirometry, Pulmonary function tests, Pulmonary evaluation, Surgery.
Spirometry is the simplest test to measure lung mechanics [1]. Understanding of this study dates back to Galen, who discovered that the volume taken in with each breath did not vary by trapping exhaled air in a rudimentary bladder [2]. Giovanni Borelli (1681) was the first to attempt to accurately measure the volume of air inspired by a breath, through a column of water in a cylindrical tube and by measuring the volume of air displaced by the water [3]. It was John Hutchinson in 1846 who developed the first functional water spirometer. He described the terms vital capacity, tidal volume, inspiratory reserve volume, expiratory reserve volume, and residual volume [4]. Tiffeneau in 1947 described the Forced Expiratory Volume in the first second (FEV1) [5]. The British Thoracic Society defined in 1956 the FEV1/FVC ratio and the forced expiratory flow between 25 and 75% of the Forced Vital Capacity (FVC) (FEF25-75%) [6]. In 2005, the American Thoracic Society (ATS) and the European Respiratory Society (ERS) published internationally the standardization criteria for lung function tests, including technical aspects and interpretation [7]. The latest update of the technical standards for interpreting routine pulmonary tests will be published in 2022 [8].
What is the current importance of spirometry in preoperative lung evaluation?, is a question that is disturbing and of great relevance, because despite being a low cost study and known by a large number of doctors of different specialties, the anesthesiologist and the surgeon do not give it relevance, to be able to orient ourselves on the current lung state of the patient, the postoperative prognosis and even the trans and postoperative actions on the patient.
A search of the current bibliography will be made to answer the previous question, and we will perform an analysis of the spirometry from the parameters derived from it, the usefulness in the perioperative, the report and the correct reading of it. Its fundamental utility in the obstructive lung diseases of the test and the origin of more study for the restrictive lung disease, a brief overview in resection surgery and spirometry analysis. Specifically for this report. A search was carried out in PubMed for the following keywords: “spirometry” (34,790 articles), “perioperative spirometry” (224 articles) and “perioperative spirometry, anesthesia” (77 articles).
Spirometry measures the magnitude of lung volumes and the speed with which they can be mobilized (airflow). It is represented graphically between the variables (Volume/Time or V/T curve) or between their derivatives (F/V curve). There are two types of spirometry: simple and forced. Simple spirometry measures static lung volumes, except Residual Volume (RV) and those others derived from its calculation, such as Functional Residual Capacity (FRC) and Total Lung Capacity (TLC). Forced spirometry measures dynamic lung volumes [9].
The National Institute for Health and Care Excellence (NICE) guidance on preoperative testing does not recommend routine preoperative spirometry for any group of patients, only makes the dimension that it should be requested after assessment by a trained anesthesiologist (thoracic anesthesiologist). In practice, if a patient is classified as ASA 3 or 4 due to respiratory illness, it is likely that they have already been evaluated by specialists and have previous spirometry. If not, it may be helpful to perform spirometry according to the numbered directions below [10].
Indications and contraindications: When the anesthesiologist decides to request a spirometry, the main indications vary somewhat with respect to the indications for general medicine, internal medicine, pulmonology, occupational medicine, or epidemiology [12].
The following indications could be numbered perioperatively: 1. Preoperative risk assessment [13]; 2. Obstruction reversibility [8]; 3. Operability and resectability criteria [14]; 4. Disease classification [15]; 5. Tidal volume calculation for thoracic surgery [16]; 6. Extrathoracic airway obstruction [17]; 7. Predicting Postoperative Pulmonary Complications (PPC) [18]; 8. Physiologic and difficult airway prediction (Table 1) [19].
| General | Perioperative |
| Evaluation of respiratory symptoms or signs | Preoperative risk assessment |
| Measurement of the effect of disease on lung function | Obstruction reversibility |
| Estimation of severity and prognosis in respiratory diseases' | Operability and resectability criterio |
| Evaluation of the effect of therapeutic interventions | Disease classification |
| Monitor the course of diseases that affect lung function | Calculation of tidal volume for thoracic surgery |
| Rutine physical exam | Extrathoracic airway obstruction |
| Epidemiological studies and research | Predict postoperative pulmonary complications |
| Probable prediction of the difficult and physiological airway |
| Absolute | |
| Hemodynamic instability | Acute coronary syndrome |
| Thoracic aortic aneurysm | Recent myocardial infarction: less than seven days |
| Pulmonary embolism | Unstable angina |
| intracranial hypertension | Preeclampsia |
| Hemoptysis | Airway infections |
| Acute retinal detachment | Acute coronary syndrome |
| Relative | |
| Children <5 years | Ophthalmic surgery |
| Altered mental state | Tracheostomy |
| Recent neurosurgery | Dental problems |
| ENT surgery | Pleural effusion |
| Abdominal surgery | Acute myocardial infarction (1 month) |
| Thoracic surgery | Nauseous state |
Spirometry must have three clearly differentiated sections: A) Demographic data of the patient, B) Spirograms (curves) and C) Environmental and technical data [22] (Figure 1). Demographic data is extremely important because the interpretation is based on the patient's sex, height, age, weight, and ethnic group, and they are compared against reference values. For Mexican American population, the most used values are the The Third National Health and Nutrition Examination Survey (NHANES III) [23]. Volume-Time (VT) and Flow-Volume (FV) spirograms should always be included in spirometry; They are very useful to assess the quality of the maneuver. These graphs show the degree of effort, its duration and the presence of artifacts; they can also serve interpretation purposes (Figure 1 and 2) [24]. Lastly, environmental and technical data are essential to corroborate spirometer calibration [25].
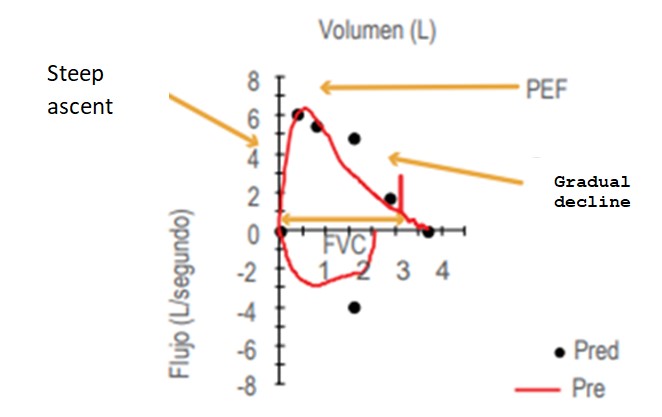 Figure 2: Flow-volume curve. Start and end acceptability criteria are observed; artifact-free maneuver (PEF=peak expiratory flow).
Figure 2: Flow-volume curve. Start and end acceptability criteria are observed; artifact-free maneuver (PEF=peak expiratory flow).
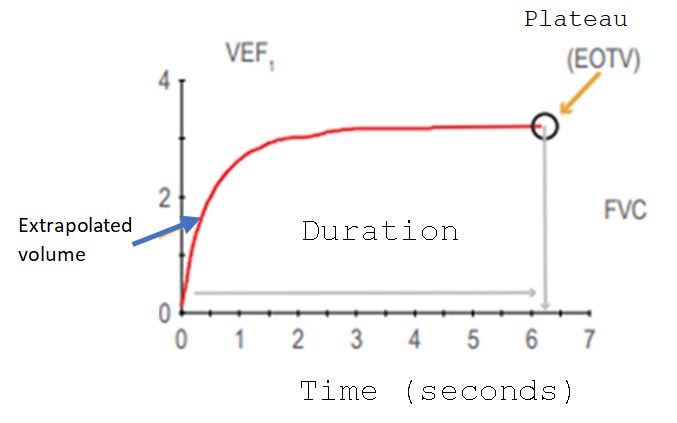 Figure 3: Volume-time curve. Start and end acceptability criteria are observed; artifact-free maneuver (FEV1=Forced Expiratory Volume in the first second, FVC=Forced Vital Capacity, EOTV=End-Expiratory Volume).
Figure 3: Volume-time curve. Start and end acceptability criteria are observed; artifact-free maneuver (FEV1=Forced Expiratory Volume in the first second, FVC=Forced Vital Capacity, EOTV=End-Expiratory Volume).
For the interpretation of spirometry to be correct by the anesthesiologist, 3 acceptability criteria must be met. These criteria evaluate the start of the effort, its duration and termination, and whether the maneuvers are free of artifacts. For them to be correct, the patient must make a maximum of eight efforts with an interval of one to two minutes each to avoid complications [26].
Repeatability refers to the greatest coincidence between results obtained from successive measurements involving the same method, same observer, same instrument, same place, same condition, repeated over a short period of time. With three acceptable maneuvers, it is necessary to verify that they are repeatable. There must be a difference of less than 150 mL (0.15 L) in adults between the highest values of FEV1 and FVC, regardless of whether they belong to different efforts.
Once our tests are acceptable and repeatable, we proceed to qualify the spirometry to report it in our pre-anesthetic assessment or in the follow-up note Table 3 [27].
| Quality grade | Acceptable maneuvers | Repeatability | Interpretation |
| A | 3 | <150 mL | Very acceptable and repeatable |
| B | 3 | <200 mL | Acceptable and repeatable |
| C | 2 | <200 mL | Less acceptable and repeatable |
| D | 2 | >200 mL | Less acceptable and variable |
| E | 1 | Inadequate | |
| F | 0 | Inadequate |
Preoperative risk assessment: The anesthesiologist in his daily practice will face multiple pathologies that involve a risk of developing Postoperative Pulmonary Complications (PPC). Asthma and Chronic Obstructive Pulmonary Disease (COPD) are common in Mexico. According to the World Health Organization (WHO) 7% of the population suffers from asthma [28] and 7.8% of the population over 40 years of age suffers from some degree of COPD [29]. CPPs are the second cause of postoperative morbidity after surgical wound infection [30]. The incidence of CPP varies according to the consulted bibliography, it can range from <1% to 23% [31,32], the most common being respiratory failure [33]. Here lies the importance of evaluating and knowing the degree of affection of these diseases. According to Postman, et al. [34] both asthma and COPD can behave with a superimposed syndrome, therefore, the FEV1 value is decreased by 50% at 60 years. It must be remembered that the peripheral airways of COPD patients, compared with normal peripheral airways, are airflow limited due to a variable combination of loss of alveolar attachments, inflammatory airway obstruction, and luminal obstruction. with mucus [35] and to know this degree of obstruction it is necessary to have a preoperative spirometry.
Reversibility of the obstruction: According to the Global Initiative for Obstructive Lung Disease (GOLD) bronchodilator reversibility should be performed during the initial evaluation to rule out the diagnosis of asthma, establish the best possible lung function for the patient, and determine the patient's prognosis [36]. β2-Adrenergic receptor agonists, such as salbutamol, are one of the first medications used by the anesthesiologist in the setting of airway obstruction (bronchospasm) [37], but when dealing with a patient at risk of bronchospasm, We must bear in mind that the use of this type of medication depends on the percentage of reversibility. According to the latest update of the European Respiratory Society / American Thoracic Society ERS/ATS guidelines, a significant response to a bronchodilator is defined as a percentage improvement of 10% in FEV1 [8].
Operability and resectability criteria: There is a clear correlation between the extent of resection and postoperative morbidity and mortality [38]. The greater the resection, this correlation increases: pneumonectomy 5.7%, bilobectomy 4.4%, segmental or wedge resections 1.4% [39]. The most used parameters in the different published works are the Vital Capacity (CV) and the Forced Expiratory Volume in the first second (FEV1 ). A CV greater than 2 liters (L) appears to be safe for lung resections [40]. One of the first studies that used FEV1 to predict complications according to the extension of the resection was the one carried out by Loddenkemper et al [41], where the measurement of FEV1 in liters for each type of resection was proposed; found that for a pneumonectomy a FEV1 >2.5 L decreased the CPP; for lobectomy >1.75 L and for wedge or segmental resections >1.5 L.
Once the resection is performed, every patient is at risk of performing some PPC. Of the various existing tests to assess respiratory mechanics, the most studied to determine the appearance of PPC after lung resection is the Postoperative Forced Expiratory Volume in 1 second (FEV1 pop) [42]. The most commonly used formula is based on the lung segments resected and the percentage of FEV1 provided by each lung [14,43]. Calculating the percentage of pop FEV1 is radically important because, according to the percentage obtained, our patient can be extubated without problems in the operating room or will have to undergo rehabilitation therapy or even be transferred to intubated intensity therapy to condition the patient's mechanical ventilation. Kearney et al. found FEV1 POP as the only independent predictor of postoperative complications [44]. Figure 4 shows the number of segments that each lung has, its contribution in % to FEV1 , the formula for calculating pop FEV1 , and the pop FEV1 management algorithm.
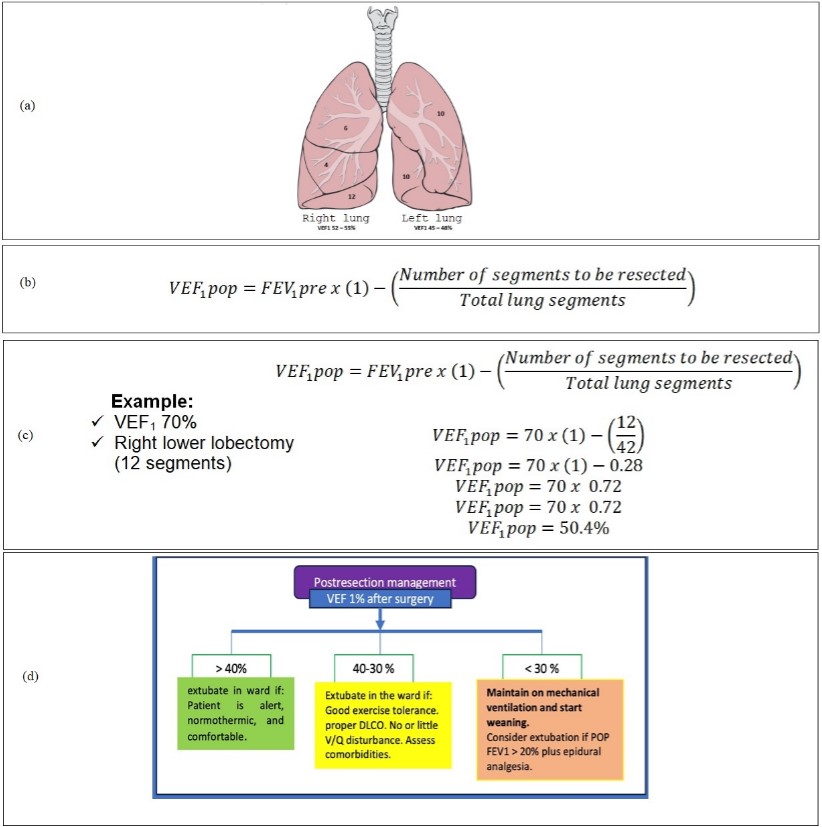 Figure 4: Calculation of pop FEV1 and lung segmental anatomy. A): Segmental lung anatomy; B): Formula for calculating pop FEV1; C): Example of calculating pop FEV1; D): Management algorithm according to the FEV1 pop obtained.
Figure 4: Calculation of pop FEV1 and lung segmental anatomy. A): Segmental lung anatomy; B): Formula for calculating pop FEV1; C): Example of calculating pop FEV1; D): Management algorithm according to the FEV1 pop obtained.
Classification of the disease: The obstructive and restrictive lung disease present different degrees of involvement, which are measured according to the FEV1 and FVC figures with respect to their reference values. The obstruction lung disease is probably the one that is of interest to the anesthesiologist. For this, it is necessary to graduate the degree of obstruction according to the FEV1 value. When grading with this value we obtain: mild obstruction with a FEV1 value of 100-70%, moderate obstruction of 65-60%, moderately severe obstruction of 59-50%, severe obstruction of 49-35%, and very severe obstruction <35% [8].
Calculation of tidal volume for thoracic surgery: The calculation of the tidal volume (Vt) is of vital importance for single lung surgery [45]. For its calculation, guidelines have been established for when ventilation has to be one-lung, with Vt being between 5-6 mL/Kg [46] and even 4 mL/Kg [47]. Recently, the use of forced spirometry has been proposed to calculate Vt. In 2017, Hoftman et al [16] determined the calculation of Vt for one-lung ventilation using the formula Vt=FVC/8 when the candidate for thoracic surgery has low compliance, analyzing 3470 patients.
Obstruction of the extrathoracic airway: Sleep Disorders (SD) affect the quality of life and some of them are the cause of morbidity and premature mortality. In Mexico, 27.3% of adults with some symptom associated with sleep have a high risk of suffering from Obstructive Sleep Apnea Syndrome (OSAS) [48]. The flow volume curve may be of interest in the diagnosis of obstructive sleep apnea syndromes by showing obstruction of the extrathoracic airways [49]. A study analyzing 57 patients found that the ratio of Expiratory Flow to Inspiratory Flow at 50 percent of the forced vital capacity (FEF50/FIF50) and the appearance of the sawtooth sign in the volume/flow curve are not exclusive of OSAS, but favor the hypothesis of a central component in the mechanism of upper airway occlusion during sleep [50].
Predict Postoperative Pulmonary Complications (PPC): PPCs are a common cause of mortality and morbidity [31]. The pioneering study by Kocabas et al [18] determined that the use of perioperative spirometry is a useful tool to predict PPC when used in conjunction with the clinic. In this way, in 2017, a retrospective observational study was published where 898 patients undergoing surgery were analyzed, finding that an FVC lower than the reference value predicts PPC with an OR of 0.98 with a CI of 95% 0.97-0.99, p=0.018 [51].
Probable prediction of the difficult (and physiological) airway: A pilot study tried to find an association between difficult laryngoscopy and spirometry [19]. It was found that a FVC less than 3.1 L under a linear regression model, is the most significant spirometry parameter to predict difficult airway (DA), with an AUC of 0.754, sensitivity of 71%, and specificity of 71%. In this way we have one more tool for the prediction of DA, this study shows an interesting association between abnormal spirometric values and visualization of the glottis difficult in direct laryngoscopy [52]. We must remember that not only a DA is based on anatomical criteria, but also physiological variables that add extra difficulty to intubation [53].
Interpretation: The fundamental parameters for the interpretation of spirometry are FEV1 , FVC, and the FVC/FEV1 ratio, which are compared with reference or predicted values. To facilitate interpretation, it is necessary that the spirometric report indicates the Lower Limit of Normality (LLN), which is defined as the 5th percentile of the reference population for any of the above indexes. It has been proposed as a way of relating any data with that of the population from which it comes, especially in those that are absolute values and not a percentage with respect to the theoretical, and specifically in the FEV1 / FVC ratio, although it has also been used with FVC, FEV1 , and total lung capacity [54]. For its correct and simple interpretation, we elaborated in Figure 5 an interpretation scheme so that any anesthesiologist can carry out an adequate reading [55]. It is important to note that spirometry is only one part of the diagnostic scheme and algorithm for respiratory disorders that can be evaluated with pulmonary function tests (Figure 6) [56].
Spirometry is an undervalued tool for anesthesiologists. This lies in the null teaching of its usefulness and interpretation. The teaching of this diagnostic test should be encouraged in the training of anesthesiology medical personnel. As experts in the airway, we must master its interpretation, in the same way that a cardiologist interprets an electrocardiogram. Spirometry should be part of the practice of every anesthesiologist.
The authors would like to acknowledge Dr. Maria Luisa Segura Fernández who supported us in the translation of the article.
We have no known conflict of interest to disclose.
No
