 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Drashti Patel1, Jonathan Benjamin1, Aashay Patel1, Chance Fleeting1, Jed Casauay1, Marco Foreman1, Sohum Sheth1, Brandon Lucke-Wold2*
1University of Florida, College of Medicine, Gainesville, Florida, USA.
2University of Florida, Department of Neurosurgery, Gainesville, Florida, USA.
Correspondence to: Brandon Lucke-Wold, University of Florida, Department of Neurosurgery, Gainesville, Florida, USA.
Received date: May 02, 2023; Accepted date: May 27, 2023; Published date: June 03, 2023
Citation: Patel D, Benjamin J, Patel A, et al. Neurostimulation for Spinal Lesions: Enhancing Recovery and Axonal Regeneration. J Med Res Surg. 2023;4(3):46-57. doi: 10.52916/jmrs234107
Copyright: ©2023 Patel D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Spinal neurostimulation is a promising approach for treating spinal lesions and has implications in various neurological disorders. It promotes axonal regeneration and neuronal plasticity to reestablish disrupted signal transduction pathways following spinal injuries or degeneration. This paper reviews the current technology and its differing utilities in various types of neurostimulation, including invasive and noninvasive methods. The paper also explores the efficacy of spinal compression and decompression therapy, with a primary focus on degenerative spinal disorders. Moreover, the potential of spinal neurostimulation in therapies for motor disorders, such as Parkinson's disease and demyelinating disorders, is discussed. Finally, the paper examines the changing guidelines of use for spinal neurostimulation following surgical tumor resection. The review suggests that spinal neurostimulation is a promising therapy for axonal regeneration in spinal lesions. This paper concludes that future research should focus on the long-term effects and safety of these existing technologies, optimizing the use of spinal neurostimulation to enhance recovery and exploring its potential for other neurological disorders.
Neurostimulation, Spinal lesions, Spinal decompression, Spinal fusion, Chronic pain, Parkinson’s disease, Demyelinating disorders, Tumor resection
tsESS: Transcutaneous Electric Stimulation of the Spine; MISCI: Motor-Incomplete Spinal Cord Injury; tsDCS: Trans-Spinal Direct Current Stimulation; mA: Milliampere; SCI: Spinal Cord Injury; TsMSS: Magnetic Trans-Spinal Stimulation; SCS: Spinal Cord Stimulation; ALIF: Anterior Lumbar Interbody Fusion; PLIF: Posterior Lumbar Interbody Fusion; TLIF: Transforaminal Lumbar Interbody Fusion; XLIF: Extreme Lateral Interbody Fusion; AxialLIF: Axial Lumbar Interbody Fusion; MIS: Minimally Invasive Spine; MIS-TLIF: Minimally Invasive Transforaminal Lumbar Interbody Fusion; NSD: Non-Surgical Spinal Decompression Therapy; SDT: Spinal Decompression Therapy; CSE: Core Stabilization Exercises; LE-ULBD: Unilateral Laminotomy Bilateral Decompression; Endo-ULBD: Endoscopic Unilateral Laminotomy; MS: Multiple Sclerosis; CMT: Charcot-Marie-Tooth Disease; ALS: Amyotrophic Lateral Sclerosis; TSS: Transcutaneous Spinal Stimulation; TA: Tibialis Anterior; TENS: Transcutaneous Electrical Nerve Stimulation; IFC: Interferential Currents; cTSS: Cervical Transcutaneous Spinal Stimulation; PD: Parkinson’s Disease; FDA: U.S. Food and Drug Administration; DBS: Deep Brain Stimulation; BurstDR: Burst Paradigm Spinal Cord Stimulation; VAS: Visual Analogue Scale; UPDRS: Unified Parkinson’s Disease Rating Scale; ADL: Activities Of Daily Living; PPP: Persistent Post-Surgical Pain; WDR: Wide Dynamic Range; GABA: γ-Amino-Butyric Acid; MRI: Magnetic Resonance Imaging; ASPN: American Society of Pain and Neuroscience
Spinal lesions can cause significant physical, psychological, and financial burdens for patients [1]. They often lead to an irreversible loss of motor, sensory, or autonomic functions, resulting in sensorimotor deficits, neuropathic pain, autonomic dysreflexia, and bladder and bowel dysfunction [2,3]. This loss of function is caused by damage to axons, which disrupts signal transduction pathways. The resulting impairment of connections between neurons leads to the disruption of ion channels, neurogenic shock, and a myriad of other inflammatory and immune responses [2,4,5]. Although axons have some capacity to regenerate, recovery from spinal disruption is rare, making most forms of spinal damage irreversible. Over the years, various areas of research within neurosurgery have increasingly focused on axonal regeneration, yielding therapeutic modalities, such as neurostimulation. Neurostimulation involves the delivery of electric impulses to stimulate the spinal cord and trigger responses from damaged areas, which promotes axonal regeneration and enhances neuronal plasticity. It has shown promising results in alleviating symptoms associated with spinal lesions; however, the effects of neurostimulation can vary based on the type of spinal lesion. In traumatic spinal lesions, neurostimulation promotes nerve connection recovery and axonal regeneration. Traumatic spinal lesions can be caused by a variety of medical conditions, ranging from age-related deterioration to demyelinating disorders, each of which will show varying degrees of benefits from neurostimulation. Contrastingly, in cancerous lesions, the primary purpose of neurostimulation is palliative care and pain management.
Not only do the effects of neurostimulation vary based on the type of lesion observed, but they are also impacted by the form of technology used. Notably, the efficacy of neurostimulation technology can vary depending on the location, strength, direction, and frequency of impulses delivered. The type of stimulation, the invasiveness of the procedure, and other therapies used in combination with the stimulation can lead to a wide range of benefits and toxicities in the treatment of spinal lesions. Hence, each technology and condition presents unique challenges and opportunities for improvement.
This paper aims to explore the efficacy of various forms of neurostimulation in spinal lesions. We will provide an overview of the different technologies available for neurostimulation, followed by an analysis of the effectiveness of spinal fusion and decompression therapy. Further, we will explore the impacts of spinal neurostimulation in motor disorders, including demyelinating disorders and Parkinson's disease. Finally, we will examine the efficacy of spinal neurostimulation in managing chronic pain in post-surgical tumor resection. By doing so, we hope to contribute to the growing body of research on neurostimulation and its potential to improve outcomes for patients with spinal lesions.
Neurostimulation is a constantly evolving field that has led to the development of numerous novel technologies in recent years. Each new form of stimulation provides a unique approach to treatment that has the potential to deliver distinct benefits for different types of spinal lesions. Currently, the primary focus of neurostimulation relates to cranial and spinal modalities, and the primary objective of this paper is to review advancements in spinal neurostimulation. There are two major categories into which spinal neurostimulation techniques can be classified: noninvasive methods and surgical stimulation [6]. As indicated by the name, noninvasive methods do not require surgical implantation to achieve their stimulatory effects on the spine. In contrast, surgical stimulation requires an invasive methodology to directly implant a neurostimulation device in the spinal cord. Both have varying degrees of utility for the treatment of spinal lesions.
Noninvasive methods: Methods for noninvasive spinal neurostimulation include both electrical stimulation and magnetic stimulation. As part of the former category, Transcutaneous Electric Stimulation of the Spine (tsESS) involves the noninvasive placement of skin electrodes to deliver electrical currents to the spine [7,8]. The premise of tsESS is that electrical stimulation can selectively activate sensory afferent neurons located in the posterior column of the spine. Stimulation of posterior column fibers can result in the subsequent activation of anterior root fibers, particularly in the lower limbs, as part of a motor reflex [9-11]. Due to this potential evocation of motor function, tsESS has been studied as a potential therapy for patients suffering from Motor-Incomplete Spinal Cord Injury (MISCI), which typically occurs following spinal trauma. One randomized, sham-controlled study involving MISCI determined that tsESS combined with locomotor training significantly improved walking outcomes when compared to sham treatments. Outcomes were measured using walking speed and 2-minute walking distance, and a reduction in spasticity was not observed [12]. Although these results are promising, tsESS has yet to prove its efficacy as a first-line treatment for MISCI. The authors of the study stated that tsESS therapy may be potentially limited in effect to treatment during the subacute phase of injury, where the highest level of neuroplasticity is observed [12,13].
Another electrical neurostimulation method currently being studied is Trans-Spinal Direct Current Stimulation (tsDCS). This procedure entails placing an electrode over a vertebral spinal process and another reference electrode on a shoulder or upper limb (Figure 1) [14]. A constant direct current electrical stimulator is applied over these electrodes, with the intensity ranging from 1.5 to 2.5 mA [7,14]. Studies involving healthy humans demonstrated that tsDCS reduces somatosensory-evoked potentials, which are associated with the lemniscal pathway, and potentials elucidated in the spinothalamic tract, which transmit pain reception to the brain [15]. Further, another study involving patients with severe Spinal Cord Injury (SCI) determined that the application of tsDCS modulates the behavior of spinal reflexes to comparable levels observed with Lokomat-driven gait orthosis [16]. Spinal reflexes are a marker of spinal neuronal dysfunction and indicate an unbalance of inhibitory and excitatory interneuronal activity. Although the exact mechanism by which this occurs remains unknown, this study provides preliminary evidence that supports the notion of adding tsDCS as a neurostimulation option for SCI treatment [17].
Lastly, magnetic Trans-Spinal Stimulation (TsMSS) is another technology available for noninvasive neurostimulation. This modality generates repeated, fluctuating magnetic fields that deliver stimulatory currents to nervous tissue, notably the cervical or thoracic spine [7]. The delivery of magnetic fields to nervous tissue has been extensively studied, and it is believed that these fields can exert neuroprotective effects on tissues [17-19]. Additionally, other studies observed that magnetic field application induces nervous tissue remodeling through the action of adult neural stem cell proliferation [17,20]. In comparison to its electric counterparts, magnetic stimulation focuses on tissue structure rather than stimulating individual neural groups. One study by Chalfouh et al. investigated the effects of TsMSS treatment on mice models for SCI [17]. The results of this study demonstrated that TsMSS application post-SCI decreases the demyelination process, thus promoting neuronal survival and leading to an increase in axonal regrowth. These results were corroborated by another study conducted in a rat animal model, which demonstrated an increase in axonal survival with TsMSS use [21]. This study also observed a decrease in cystic cavities, a phenomenon observed with human SCI. There is limited literature available regarding the use of TsMSS in human SCI; however, these preclinical studies provide promising evidence that TsMSS can potentially serve a prominent role in treating SCI.
Surgical neurostimulation: Surgical neurostimulation primarily exists through a procedure known as Spinal Cord Stimulation (SCS) [22]. Although SCS is more invasive than the aforementioned techniques, it is still a minimally invasive procedure [23]. Currently, SCS has demonstrated potential in treating pharmacoresistant chronic pain and is considered a more attractive alternative than nerve ablation [6,22,23]. This procedure entails placing electric stimulators in the epidural space of the spine, which allows the electrodes to deliver current to the spinal dorsal columns [24]. SCS is a generally acceptable option for patients experiencing radicular pain in their lower extremities. It is also indicated for patients who cannot undergo other surgical spinal procedures to alleviate pain following failed back surgery syndrome [23]. Recent patient cases involving spinal intradural tumors also demonstrate the efficacy of SCS in managing chronic pain as a result of tumor resections; although, the exact placement of electrodes to maximize benefits has yet to be determined [25]. Additionally, there are special considerations for deciding the candidacy of patients for SCS. For example, patients with pacemakers or defibrillators need close cardiology follow-up when undergoing SCS placement, and patients with thrombocytopenia are contraindicated for SCS placement due to the higher risk of developing spinal epidural hematoma [26].
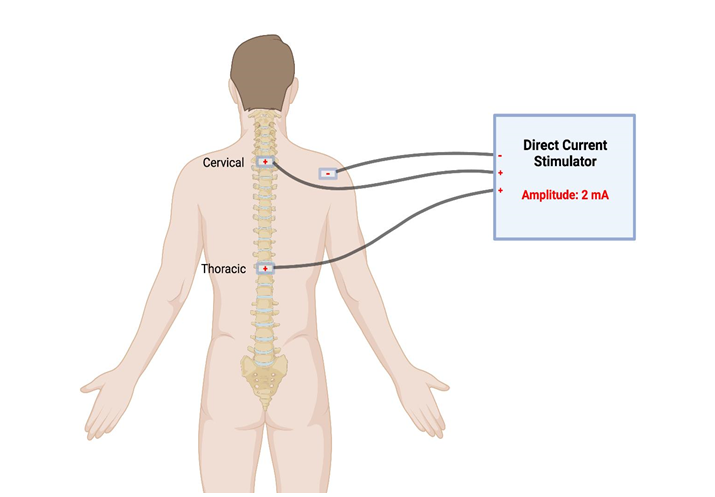 Figure 1: tsDCS involves electrodes placed superficially on the skin over the locations of vertebral spinal processes. These electrodes can either be near the cervical spine or thoracic spine, depending on the location of the patient’s spinal lesion. In this example, the reference electrode is placed on the right shoulder. Figure created with BioRender.com, accessed on 11 April 2023.
Figure 1: tsDCS involves electrodes placed superficially on the skin over the locations of vertebral spinal processes. These electrodes can either be near the cervical spine or thoracic spine, depending on the location of the patient’s spinal lesion. In this example, the reference electrode is placed on the right shoulder. Figure created with BioRender.com, accessed on 11 April 2023.
In addition to spinal neurostimulation techniques for spinal lesions, spinal fusion and spinal decompression therapy can be used for various forms of damage to the spinal cord. They are often utilized after conservative methods for pain management or spinal dysfunction have proven unsuccessful for a patient [27]. Spinal fusion techniques involve the fusion or joining of two or more vertebrae; whereas, spinal decompression aims to decrease pressure on the spinal cord by creating space between two vertebrae. Both techniques involve evolving technologies and have different implications for use, as discussed below. Spinal fusion therapy: Spinal fusion therapy is predominantly indicated for use in spinal degeneration, which is often related to the aging process. It currently serves as a treatment for scoliosis, cervical myelopathy, and lumbar back pain, and it is further being researched for its utility in pediatric populations for postoperative pain management in scoliosis or bone grafting [28,29]. The traditional method of achieving spinal fusion is through open spine surgery, often of the lumbar region.
For lumbar spinal surgery, open spinal fusion can be performed by either an Anterior Lumbar Interbody Fusion (ALIF) or Posterior Lumbar Interbody Fusion (PLIF) approach [30]. Several studies evaluated the outcomes of both approaches in order to provide surgeons with information for pre-operative planning. These studies found that ALIF was associated with an overall increased hospital length of stay and a higher mortality rate than PLIF [31,32]. Both approaches have therapeutic utility for several other disorders, including cervical myelopathy. Mixed data exists regarding which approach is optimal for cervical myelopathy due to variations in how outcomes are measured; however, multiple studies concluded that PLIF was associated with a longer hospital length of stay and higher rates of inpatient complications than ALIF [33,34]. Hence, ALIF and PLIF have distinct benefits and complications for different disorders; therefore, the outcomes of both should be compared to select the best option for spinal fusion therapy.
The beneficial impacts of these modalities have led to the development of a Transforaminal Lumbar Interbody Fusion (TLIF) approach. TLIF has the potential to be therapeutically valuable in all spinal degenerative pathologies [27]. After TLIF was determined to be efficacious, Extreme Lateral Interbody Fusion (XLIF) and Axial Lumbar Interbody Fusion (AxialLIF) techniques became popularized and are now also utilized in spinal fusion procedures [35]. Another form of spinal fusion therapy that recently arose is Minimally Invasive Spine (MIS) surgery. First introduced in 2003, MIS has become increasingly popular for spinal fusion techniques due to improved safety and reduced postoperative complications [36]. MIS surgery is performed via the transforaminal approach, known as the Minimally Invasive Transforaminal Lumbar Interbody Fusion (MIS-TLIF). This approach is praised for the short-term outcomes of reduced operative blood loss, postoperative pain, and hospital length of stay when compared to open TLIF. Long-term outcomes were similar between the two approaches, with no significant difference noted [37].
Although spinal fusion is typically indicated as a minimally invasive treatment for various pathologies, the risk of associated complications must be taken into consideration before recommending this option to patients. For instance, one drawback to MIS-TLIF is increased exposure to radiation for both the patient and surgeon during the procedure [38]. Several studies have also investigated the efficacy of spinal fusion for lumbar back pain associated with degeneration and concluded that the benefits of spinal fusion compared to non-operative treatment remain unclear [39]. Moreover, no prognostic tests are recommended for selecting patients for lumbar spinal fusion, which makes identifying candidates a very difficult process [40]. Despite the changing guidelines for use, multiple studies have shown countless benefits of spinal fusion therapy for chronic pain management and degenerative diseases. Spinal decompression therapy: Similar to spinal fusion therapy, spinal decompression therapy is currently indicated for use in alleviating spinal nerve pain and spinal cord damage that results from aging. It is also a part of the traditional approach to spinal tumor resection. Spinal decompression can be achieved by various non-surgical and surgical methods, which are examined below.
Non-surgical methods for spinal decompression involve two prominent techniques: traction therapy and Non-Surgical Spinal Decompression Therapy (NSD). Few studies have championed the effectiveness of traction therapy; furthermore, the validity of these studies has been questioned by other researchers. Contrastingly, multiple validated studies have pointed out that traction therapy is ineffective [41,42]. In the case of NSD, one study suggested that NSD in combination with physical therapy leads to better results than physical therapy alone for patients with lumbar radiculopathy [42]. NSD in combination with core stabilization exercises also yielded better outcomes than the core stabilization exercises alone for patients with lumbar disc prolapse [43]. These findings suggest that NSD can be a beneficial treatment option, but further research is needed to compare NSD to other conservative decompression treatments. When comparing patient outcomes of NSD to general traction therapy, Choi et al. found no statistically significant difference in their efficacy [44].
Spinal decompression can also be achieved through surgical intervention. Surgical decompression techniques can be further divided into indirect and direct procedures. Indirect spinal decompression involves decompressing spinal nerves without the resection of faulty tissue [45]. One example of this technique is ligamentotaxis, which has been proven effective for treating compression and burst fractures [46]. Common direct techniques for posterior spinal decompression include open laminectomy, hemilaminectomy, laminotomies, and laminoplasty [47]. Spinal laminectomy is one of the most common procedures used to decompress structures in the spine. It involves the removal of the spinous process and lamina and is commonly used to treat spinal stenosis. It is also a component of the traditional approach for spinal tumor resection, which is the most effective treatment for benign spinal tumors [47-49]. One common complication of posterior decompression for intramedullary tumor removal is spinal deformity [45]. To reduce the risk of spinal deformity and instability, spinal fusion can be performed after the laminectomy [47].
One alternative to open laminectomy is minimally invasive posterior decompression. Procedures for minimally invasive posterior decompression include unilateral laminotomy, bilateral laminotomy, and spinous process osteotomy [50]. Similar to other procedures, such as MIS-TLIF, minimally invasive posterior decompression can be used to treat spinal stenosis with similar outcomes [50,51]. Overall, many forms of spinal decompression therapy have proven to have distinct benefits for various spinal abnormalities. Given the wide range of options available for spinal decompression, Table 1 lists several studies identified that compare two or more decompression techniques and their outcomes.
|
Study |
Disorder |
Treatment options compared |
Patients enrolled in the study |
Optimal treatment |
|
Engelhard H, et al. 2010 |
Primary tumor of spinal cord |
Resection (via laminectomy [92.7%], biopsy, radiosurgery, or endoscopic surgery), chemotherapy, and radiation |
430 patients; Resection/biopsy performed in 89.3% of cases, radiation therapy, and chemotherapy were administered to 20.3% and 5.6% of patients respectively |
Tumor resection via laminectomy. |
|
Gaowgzeh RAM, et al. 2020 |
Lumbar disc prolapse |
Spinal decompression therapy (SDT) and core stabilization exercises (CSE) vs CSE alone |
31 patients; SDT and CSE (n=16), CSE (N=15) |
Spinal decompression therapy and CSE yield higher outcomes when compared to CSE alone. |
|
Hermansen E, et al. 2022 |
Lumbar stenosis |
Unilateral laminotomy, bilateral laminotomy, or spinous process osteotomy |
437 patients; unilateral laminotomy |
No clinically significant difference in outcomes between unilateral laminotomy, bilateral laminotomy, or spinous process osteotomy. |
|
(n=146), bilateral laminotomy |
||||
|
(n=142), spinous process osteotomy (n=149) |
||||
|
Hua W, et al. 2020 |
One-level lumbar stenosis |
Unilateral laminotomy bilateral decompression (LE-ULBD) versus minimally invasive surgery transforaminal lumbar interbody fusion (MIS-TLIF) |
112 patients; LE-ULBD (n=32), MIS-TLIF (n=80) |
No clinically significant difference in patient outcomes between LE-ULBD and MIS-TLIF. LE-ULBD was associated with lower healthcare costs. |
|
Wei FL, et al. 2021 |
Lumbar stenosis |
Laminotomy, decompression, decompression plus fusion, endoscopic decompression, interspinous process spacer device implantation, laminectomy, laminotomy, and minimally invasive decompression |
4341 patients across 34 trials |
No significant clinical difference in patient outcomes between different interventions. |
|
Yuan W, et al. 2013 |
Cervical spondylotic myelopathy |
Laminoplasty versus skip laminectomy |
224 patients; Laminoplasty |
Skip laminectomy had favorable outcomes and led to improved range of motion, less complications, and less surgical trauma when compared to laminoplasty. |
|
(n=110), skip laminectomy |
||||
|
(n=114) |
||||
|
Zhao XB, et al. 2021 |
Lumbar spinal stenosis |
Percutaneous endoscopic unilateral laminotomy (Endo‐ULBD) and bilateral decompression versus PLIF |
69 patients; Endo-ULBD |
Endo-ULBD had better early (1-day post-operative) outcomes compared to PLIF. However, there was no clinically significant difference in outcomes of patients treated with Endo-ULBD or PLIF at final follow up (> 6 months post-operatively). |
|
(n=31), PLIF |
||||
|
(n=38) |
Generally, patient outcomes did not differ significantly between different techniques. However, minimally invasive approaches were noted to have less early postoperative pain and blood loss during the procedure [43,49-54].
Movement disorders are neurological conditions that lead to pathologic changes in voluntary or involuntary movement [55]. Because movement disorders are often a result of the demyelination of axons or a loss of neurons, neurostimulation can be used to prevent disease progression, manage pain, or improve motor coordination. Neurostimulation has been implicated for use in demyelinating disorders, such as Multiple Sclerosis (MS), Charcot-Marie-Tooth (CMT) disease, and Amyotrophic Lateral Sclerosis (ALS). It is also being further studied for its utility in Parkinson’s disease.
Multiple sclerosis: Multiple Sclerosis (MS) is characterized by chronic autoimmune demyelination of the central nervous system, which can lead to progressive sensorimotor impairments, neuropathic pain, muscle weakness, and fatigue. MS is the most frequently occurring type of neuronal demyelination, and its high prevalence combined with the chronic, debilitative nature of MS progression highlights the need for novel therapeutic approaches to treat resultant MS-related neurological weaknesses. One such therapeutic approach is neurostimulation. Multiple studies have shown its efficacy in improving various quality of life and functional performance metrics in MS patients.
One metric that showed improvement with neurostimulation in patients with MS was postural stability. Roberts et al. investigated the effect of non-invasive Transcutaneous Spinal Stimulation (TSS) on postural stability during upright standing. The study measured the center of pressure displacement and electromyograms from the soleus and Tibialis Anterior (TA) standing in the presence and absence of non-invasive TSS in seven individuals with MS. The investigators found that TSS significantly improved postural stability with closed eyes, which was further supported by a decrease in TA activity with TSS when compared to no stimulation [56]. Postural stability, however, was not found to significantly improve with eyes open, highlighting one limitation of this modality.
Aside from sensorimotor capabilities, other studies have demonstrated the effect of neurostimulation on chronic pain in patients with MS. In one study, 40 patients with chronic pain were divided into two groups, with one group receiving Transcutaneous Electrical Nerve Stimulation (TENS) and the other group receiving Interferential Currents (IFC). Afterward, their pain severity, quality of life, and functional capacity were measured, using questionnaires for the two former matrices and a two-minute walk test for the latter metric. Both groups showed a significant decrease in pain severity, an increase in quality of life, and an increase in functional capacity via the two minute walk test. Further, no significant difference was found between the two stimulation groups [57]. Neurostimulation through spinal cord stimulation was also studied in a patient with concurrent MS and failed back surgery syndrome, resulting in intractable pain. After treatment, the patient reported a significant improvement in pain scores and experienced a return to day-to-day activities and a reduction in analgesic medication consumption, highlighting an additional efficacious use for neurostimulation in patients with MS. Similarly, Provenzano, et al. studied a patient that underwent spinal cord stimulation for neuropathic pain and functional limitations associated with MS. At a 24-month follow up, the patient reported a drastic improvement in pain, and a significant reduction in analgesic use and spasticity levels [58].
Charcot-marie-tooth disease: SCS is also implicated in helping treat intractable pain in other neuropathies, such as Charcot-Marie-Tooth (CMT) disease, a hereditary sensory and motor neuropathy often associated with moderate to severe chronic extremity pain [59]. In a case study of a 37-year-old patient with CMT implanted with an SCS device, patient pain and quality of life were assessed at one- and six-month intervals post-implantation. SCS was found to be effective in improving quality of life and decreasing pain and medication consumption [59]. The demonstrated efficacy of SCS for pain reduction in CMT highlights the breadth of therapeutic targets for SCS.
Amyotrophic lateral sclerosis: Other neurostimulation modalities have also been investigated for their potential in treating the symptoms of Amyotrophic Lateral Sclerosis (ALS), a chronic neurodegenerative disease for which there is currently no cure. For instance, one study by Wu, et al. examined the utility of cervical Transcutaneous Spinal Stimulation (cTSS) in patients with ALS or spinal cord injury. A novel configuration for cTSS that involved the placement of electrodes over the midline in the posterior T2-T4 and anterior C4-C5 levels was developed and tested; resultant electromyographic responses were measured in arm and hand muscles [60]. This method of posteroanterior cTSS was demonstrated to be capable of activating motor neurons in upper extremity muscles whilst also being well tolerated in these subjects. Although cTSS showed promise in addressing upper extremity muscle weakness in ALS patients, one case study by Lazzaro, et al. demonstrated the potential limitations of neurostimulation in treating ALS progression. The patient in this case study was a 75-year-old male implanted with cervical SCS following ALS diagnosis; he was experiencing lower limb weakness and upper extremity denervation. SCS was effective for pain control in this patient, but ALS disease progression remained unchanged when compared to disease progression ratings before the stimulation was applied. ALS disease progression was measured using the ALS functional rating scale [61]. The small sample size of this study precludes its ability to conclude that SCS is ineffective in treating ALS disease progression, but the reduction in pain experienced by this patient post-SCS implantation is concurrent with other aforementioned studies, conveying its utility in pain management.
Parkinson’s disease: Towards the end of the twentieth century [62], interest in neurostimulation-based therapy began to accumulate with regard to the treatment of Parkinson's Disease (PD) and the maintenance of Parkinsonian symptoms. In 2003, the FDA approved Deep Brain Stimulation (DBS) as a treatment for PD [63]. Currently, DBS has not yet entered official clinical use for the direct purpose of treating PD, but investigation has begun to examine the applicability of SCS for symptomatic maintenance in severe cases of PD. Beginning in 2009 [64], SCS emerged as a potentially viable alternative to help control the otherwise unresponsive motor symptoms of PD, particularly freezing gait and bradykinesia [62]. Additionally, the effects of SCS-based pain management on the quality of life are being researched today.
Most of the current supporting evidence for the practical use of SCS in treating PD originates from animal studies, case reports, or studies limited by their control and sample size [64-71]. Most large analyses show mixed results pertaining to the efficacy of SCS in PD [70,72,73]. It should be noted that no explicitly negative or detrimental results have been revealed; the results of spinal cord stimulation for PD span from no effect to notable improvement. When traditional tonic stimulation is studied, SCS was effective in improving gait disorder, motor symptoms, and quality of life in specific cases of PD, even in the absence of chronic pain [74]. In patients with pain, SCS has shown notable positive effects when analyzed using an off-stimulation blind period of 15 days; furthermore, lasting improvement was observed in pain during 3 and 12-month follow-up visits (Figure 1), as well as a wide span of sensory and motor symptoms (Table 1) [66,75,76].
In line with these results, multiple theories on possible mechanisms of action of SCS are under review, the most prominent of which states that SCS works to desynchronize and suppress pathological beta frequency oscillations (Figure 2) [72]. Currently, due to sample size constraints and the novelty of this work, results are still widely inconclusive. As recommended by multiple sources, additional research is required to comprehensively determine efficacy, safety, therapeutic parameter ranges, and side effects, such as paresthesia [70,71,77]. Current results imply that higher frequencies (>200 Hz) seem to be more effective in improving gait than lower frequencies, but the effects of differences in pulse width and amplitude are still widely unknown. The ideal positioning of the implant and interaction with DBS is also unclear at this time [71].
Aside from future work required to establish the efficacy and feasibility of SCS, there are a few significant innovative approaches currently being developed. The intended direction that SCS for PD seems to be going in is towards non-invasive and synergistic activity, aiming to augment existing therapies such as DBS and medication. Analysis into the efficacy of transcutaneous magnetic spinal cord stimulation has started to garner attention in reference to pain management and its possible effects on Parkinsonian symptoms [78]. Additionally, novel and optimized paradigms, such as burst paradigm spinal cord stimulation (BurstDR), have shown promise in the management of Parkinsonian symptoms. Through one case study, patients with PD were treated with the BurstDR paradigm and subsequently exhibited improved motor symptoms, as well as emotional responses at a lower amplitude than was required with SCS [79]. Based on the novelty of this treatment modality, many aspects of its future applications and advancements are not yet perceptible. Looking at its current trajectory, however, SCS may soon revolutionize the treatment, and more importantly, the quality of life of patients with PD.
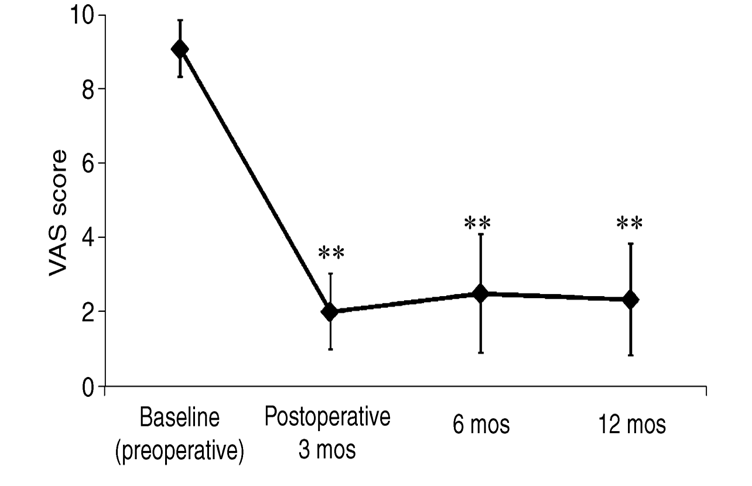 Figure 2: Changes in Visual Analogue Scale (VAS) scores of pain for 16 patients as found by Agari and Date, 2012 [76]. Immediate and lasting improvement can be observed at the 3, 6, and 12-month follow-ups with a p<0.001 (indicated by **) when compared to baseline.
Figure 2: Changes in Visual Analogue Scale (VAS) scores of pain for 16 patients as found by Agari and Date, 2012 [76]. Immediate and lasting improvement can be observed at the 3, 6, and 12-month follow-ups with a p<0.001 (indicated by **) when compared to baseline.
|
|
Baseline (preoperative) |
Postoperative 3 months |
Postoperative 12 months |
|
|
UPDRS ADL Score |
||||
|
Total |
24.0 ± 8.1 |
18.9 ± 6.8** |
20.4 ± 8.8* |
|
|
Hygiene (item 11) |
1.8 ± 0.9 |
1.7 ± 0.8 |
1.9 ± 0.9 |
|
|
Turning in bed and adjusting bed clothes (item 12) |
2.3 ± 1.0 |
1.7 ± 0.8** |
1.7 ± 0.9* |
|
|
Falling (item 13) |
1.7 ± 1.0 |
1.4 ± 0.8 |
1.7 ± 1.0 |
|
|
Freezing (item 14) |
1.0 ± 0.7 |
0.8 ± 0.6 |
1.1 ± 0.8 |
|
|
Walking (item 15) |
2.5 ± 0.6 |
1.9 ± 0.6* |
2.1 ± 0.7 |
|
|
Sensory complaints related to PD (item 16) |
3.8 ± 0.4 |
0.7 ± 0.7** |
0.7 ± 0.8** |
|
|
UPDRS Motor Score |
||||
|
Total |
23.5 ± 9.7 |
18.9 ± 10.4* |
21.3 ± 12.2 |
|
|
Rigidity (item 22) |
2.9 ± 3.3 |
2.1 ± 2.9 |
2.4 ± 2.9 |
|
|
Leg agility (item 26) |
2.3 ± 1.3 |
2.0 ± 1.3 |
2.3 ± 1.5 |
|
|
Arising from chair (item 27) |
1.7 ± 1.0 |
1.2 ± 0.9 |
1.5 ± 1.1 |
|
|
Posture (item 28) |
2.4 ± 0.9 |
1.8 ± 1.1* |
2.1 ± 1.2 |
|
|
Gait (item 29) |
2.5 ± 0.6 |
1.5 ± 0.6* |
1.8 ± 0.9* |
|
|
Postural stability (item 30) |
2.3 ± 0.6 |
1.5 ± 0.8* |
1.8 ± 1.1 |
|
|
Body bradykinesia (item 31) |
1.8 ± 0.7 |
1.5 ± 0.8* |
1.7 ± 1.0 |
|
|
Gait function |
21.6 ± 10.7 |
15.6 ± 7.3** |
18.2 ± 10.8 |
|
|
Timed Up and Go test (sec) |
14.7 ± 8.4 |
12.7 ± 8.0** |
13.3 ± 9.3* |
|
Neurostimulation has therapeutic benefits in traumatic lesions and neurodegenerative disorders, but it can further be applied to alleviate chronic pain in the context of post-surgical tumor resection. The prevalence of postoperative pain following spinal intradural tumor resection, specifically, is an overwhelmingly understated complication with up to 40% of patients suffering from chronic neuropathy [81]. Additionally, 30% of patients experience pain equal to or worse than their level of pain before the tumor resection procedure, emphasizing the severity of this issue [82]. This frequent adverse event presents a disabling postoperative sequela of spinal tumor resection that substantially impairs the quality of life for affected patients [83]. Traditionally, such post-operative, chronic neuralgia has been treated with various combinations of corticosteroids, other anti-inflammatory medications, and tumoricidal pharmaceuticals [84].
|
|
Drug Class |
Drug |
Adverse Effects |
|
First-Line Therapy |
Gabapentinoids |
Gabapentin |
Lethargy, vertigo, peripheral swelling, blurred vision |
|
Pregabalin |
Lethargy, vertigo, peripheral swelling, increased body weight |
||
|
Tricyclic antidepressants |
Amitriptyline |
Anticholinergic effects, QT prolongation (arrhythmia), suicide risk, urinary retention |
|
|
Serotonin-norepinephrine reuptake inhibitors |
Duloxetine |
Nausea, lethargy, constipation, ataxia, dry mouth |
|
|
Venlafaxine |
Nausea, vertigo, lethargy, hyperhidrosis, hypertension |
||
|
Second-Line Therapy |
Opioids |
Tramadol |
Nausea/vomiting, constipation, lethargy, seizures, ataxia |
|
Tapentadol |
Nausea/vomiting, constipation, lethargy, seizures, ataxia |
||
|
Topical treatment |
Lidocaine |
Local erythema, itching and rash |
|
|
Capsaicin |
Pain, erythema, itching; rare cases of high blood pressure |
||
|
Third-Line Therapy |
Strong opioids |
Morphine |
Nausea, vomiting, constipation, dizziness, and lethargy |
|
Neurotoxin |
Oxycodone |
Nausea/vomiting, constipation, lethargy, respiratory control |
|
|
Botulinum toxin |
Pain at injection site, toxicity |
In addition to these adverse effects, the current medications used to treat chronic pain secondary to spinal tumor resection have mechanisms that focus on treating the symptoms rather than the etiologic cause of pain. Thus, researchers and clinicians are trying to incorporate the application of neurostimulation in said patients as a step toward alleviating this neuropathic burden by addressing the pathologic condition at the precipitating source.
To understand how SCS can be applied in the setting of post-surgical tumor resection, it is crucial to understand the mechanisms underlying the transition from acute to chronic pain. The chronic pain that lingers following an acute insult, such as surgery, is known as Persistent Post-Surgical Pain (PPP); furthermore, it occurs due to the interplay between peripheral and central sensitization, as well as descending modulation [86]. In short, central and peripheral sensitization refer to the hyperexcitability of the respective nociceptors due to increased spontaneous firing and alterations in the transduction, conduction, and neurophysiology of nociceptive afferent fibers [87] Specifically, these changes, known as neuroinflammation, are the result of a cascade of events involving ion channel permeability, receptor/channel density, and gene expression [88]. Moreover, these changes are a direct consequence of when stimulus intensity reaches a noxious range over long periods of time, and their continued sensitization results in the hyperalgesia and allodynia observed in PPP following post-tumor resection [89]. Further, the dorsal spinal horn-the site of convergence for multiple complex excitatory, inhibitory, and modulatory mechanisms-serves as the interface between central and peripheral nociception, as well as the incubator for the descending modulation implicated in determining the duration of pain [86]. Accordingly, numerous experimental studies have determined SCS works via targeting overexcited Wide Dynamic Range (WDR) neurons present within the integration centers of the dorsal horn by modulating increases in γ-Amino-Butyric Acid (GABA) release [90]. This targeted mechanism is clinically significant because SCS facilitates the direct neutralization of wound-up WDR neurons, which are key players in the abnormal transmission of pain sensation to the brain [91].
The utility of neurostimulation in the context of chronic pain management following tumor resection has primarily been evaluated in case reports and studies. A recent Cochrane systematic review evaluating SCS for post-cancer pain found that SCS was able to minimize pain by 50% in more than 80% of patients; furthermore, over half of these patients reported decreased utilization of analgesic medications afterward [92]. Noordhof and colleagues also completed a case study involving a 57-year-old female with neuropathic pain in both legs following T7 level intradural meningioma resection [81]. Following T5 SCS implantation, the patient endorsed significant pain relief and minimal use of analgesics after 36 months. In another case, Brecker and Eisenberg described a 50-year-old female with foramen magnum meningioma, who was experiencing sharp pain following foramen magnum craniotomy and C1 laminectomy [93]. Interestingly, electrodes were placed at the T12 level, demonstrating that electrode placement distal to the site of the lesion can also carry significant therapeutic benefits. The patient endorsed immediate and complete pain relief; however, she was still taking 800 mg of gabapentin and using the stimulator for 16 hours each day [93]. Given the role of the dorsal column in SCS efficacy, the authors noted that an intact dorsal column is a crucial prognostic factor for distal SCS efficacy [93]. Additional case reports and case series report a similar utility of SCS in the management of chronic pain refractory to first-line treatment [94,95]. Overall, results suggest that SCS results in a reduced intensity of pain, reduced use of analgesics, and improved quality of life within this population. However, rigorous evaluation in the form of randomized controlled trials is necessary for a more robust analysis of impact.
Given the promising yet under-evaluated efficacy of neurostimulation, recent guidelines have been developed on the appropriateness of use for SCS in post-cancer chronic pain management. The Neuromodulation Appropriateness Consensus Committee recommends the utilization of conventional chronic pain treatment algorithms for cancer patients with chronic pain, which include SCS [96]. Additional considerations, however, are that SCS should be limited to those with extended remission, slow disease progression, or complete remission. Further, the future MRI needs of these patients should be considered before proceeding with SCS. The American Society of Pain and Neuroscience (ASPN) endorses SCS for patients with refractory cancer pain and pain related to cancer treatment, such as peripheral neuropathy secondary to chemotherapy [97]. However, these guidelines are at the lowest evidence levels given the lack of rigorous trials supporting the use of neurostimulation for post-cancer pain. Similarly, the European Federation of Neurological Society provides a “weak recommendation” supporting the use of SCS as a supplement to conventional medical management, given the promising results of case studies but the low quality of evidence [98]. The results of case reports following spinal tumor resection are promising and have informed various guidelines for use, but randomized controlled trials are needed for stronger recommendations to be made.
Neurostimulation has shown promising results as a therapeutic modality for patients with spinal lesions. Our investigation of the various forms of neurostimulation has demonstrated its effectiveness in promoting recovery and axonal regeneration. Current technology for spinal neurostimulation has evolved significantly over the years, with both invasive and noninvasive methods becoming increasingly researched and available in clinical use. Spinal fusion and decompression therapy have also shown promise in enhancing recovery and promoting axonal regeneration, particularly in patients with degenerative spinal lesions. In addition, our analysis of neurostimulation for motor disorders, such as demyelinating disorders and Parkinson's disease, has highlighted its potential to improve motor function and quality of life for these patients. Finally, our examination of chronic pain management in post-surgical tumor resection has shown that neurostimulation can be an effective alternative to traditional pain management strategies. However, further research is needed to fully understand the long-term efficacy and safety of these interventions, as current research for spinal neurostimulation often involves preclinical studies or case reports. Overall, the potential benefits of neurostimulation for spinal lesions are numerous, and this technology has the potential to significantly improve the quality of life for patients with spinal lesions. As research in this field continues to evolve, it is important to continue exploring new applications and technologies that can further enhance the effectiveness of neurostimulation for spinal lesions.
We have no known conflict of interest to disclose.
No
