 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Cristiano Luigi1, Pratellesi Tiziano2 1 PrestigeLab, Prestige Company, Loro Ciuffenna (AR), Italy 2 BAC Srl, Incisa e Figline Valdarno (FI), Italy
Correspondence to: Luigi Cristiano, PrestigeLab Prestige, Loro Ciuffenna, Italy; Email: prestige.infomed@gmail.com
Received date: November 18, 2020; Accepted date: December 03, 2020; Published date: December 10, 2020
Citation: Luigi C, Tiziano P (2020) Mechanisms of Action And Effects of Pulsed Electromagnetic Fields (PEMF) in Medicine. J Med Res Surg 1(6): pp. 1-4.
doi:10.52916/jmrs204033
Copyright: ©2020 Luigi C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited. Authors contribution: The authors contributed equally to the writing of this work.
Pulsed Electromagnetic Field (PEMF) therapy is a non-invasive and non-thermal treatment widely used nowadays to treat various types of disorders and traumas, both in humans and animals. Initially applied only for wound healing, today it finds many applications in medicine for the treatment of bone fractures, arthritis, inflammation, edema, and pain. Although their mechanisms of action are still being studied today, and mainly related to the calcium signaling pathway, they are effective in the adjuvant treatment of many human diseases in different medical specialties. This work aims to report the main evidence and research in the medical field with particular reference to the application of PEMF to some medical specialties as regenerative medicine (wound care), sports medicine, orthopedics, and physiotherapy. Finally, this work also wanted to deepen one of the most recent applications of PEMF in the field of complex diseases, i.e. in the adjuvant treatment of cancer. Pulsed electromagnetic field therapy may play an important role in medicine as a complementary treatment for various human diseases and, by deepening the studies in the future, it will be possible not only to understand the exact mechanisms of action but also to extend its application to other pathologies both in the medical and veterinary fields
EMF, Pulsed Electromagnetic Fields (PEMF), Wound healing, Regenerative medicine.
CaM: Calmodulin; cNOS: Cytoplasmic Nitric Oxide Synthase; NO: Nitric Oxide; PEMFs, Pulsed Electromagnetic Fields; cGMP: Cyclic Guanosine Monophosphate.
Pulsed electromagnetic field (PEMF) therapy is a type of electrotherapy that uses pulsed electromagnetic fields to treat an injured area of tissue [1]. The key to the mechanism of action of PEMF, and all its biological effects on cells and tissues, lies precisely in the modulation of the electromagnetic pulse in a pulsed, rather than the continuous manner, as in classic magnetotherapy, from which it is completely different. PEMF is not part of magnetotherapy technologies.
The device that delivers PEMFs consists of a micro-generator and an antenna. The latter is the effector part of the device, i.e. the active part that emits PEMFs with a typical carrier frequency of 27.12 MHz [2]. PEMF waveforms have been designed to penetrate completely through all types of tissues, from the skin to bone, and represent a non-invasive and non-thermal type of treatment [3]. Therefore, since their mechanism of action resides exclusively in non-thermal effects, they can be used in medicine as a complementary treatment of many types of pathologies, included traumas or disorders related to sports medicine and physiotherapy, and any type of acute inflammation or injury characterized by a high inflammatory component.
Generally, treatment times range from 20 minutes to 8 hours a day, depending on the nature of injury and characteristics of the device [4], but PEMFs can be kept active even in cycles of twenty-four hours until the attenuation and/or disappearance of the cardinal signs and symptoms of inflammation [5], including pain, the attenuation of which starts from the delivery of the first impulse by the device.
PEMF has been applied and studied for over twenty years as a non-invasive technology for the promotion and speed of wound healing and is effectively used as an adjuvant treatment in various pathologies including bone fractures, arthritis, osteoarthritis, acute inflammation, chronic inflammation, edema, pain, chronic pains, wounds, and chronic wounds [1,6- 10]. In the last ten years, their mechanisms of action have been deepened and, although they are still the subject of study today, some biochemical and metabolic pathways have been highlighted that are activated as a result of their interaction with living tissues and that explain the therapeutic effects detected in vivo, as well as in vitro.
The mechanisms of action of PEMF can be divided into three types, i.e. physical mechanisms, biophysical mechanisms, and purely biological mechanisms. While the physical mechanism of action is relatively simple, well known, and related to Faraday’s law of induction, which states how “a time-varying (pulsating) electromagnetic field induces an electric field in a nearby conductor” [1], the mechanisms of action biophysical and biological are indeed very complex.
With every single pulse, the target tissue is hit by anelectromagnetic field. The main effect of this stimulation happens at the level of the plasma membrane of the cells, which undergoes a transient depolarization [7]. This event triggers very important secondary effects (a biophysical mechanism) through the transient opening of specific transmembrane ion channels, among which the voltage-dependent channels for the Calcium ion (Ca2+) stand out. Calcium is an important second cellular messenger, in fact, the entry of calcium into the cell, and its binding at the cytoplasmic level with Calmodulin (CaM), in a time equal to milliseconds from a single pulse, triggers a whole set of biochemical pathways to cascade in the cytoplasm. They include various enzymatic activations among which the release of Nitric Oxide (NO), in a time equal to seconds from a single pulse, through the activation of the cytoplasmic nitric oxide synthase (cNOS) [1,3,7,11,12].
Nitric oxide, considered a soluble hormone, in turn, activates a whole set of biochemical pathways, one of which leads to the production of Cyclic Guanosine Monophosphate (cGMP), another second messenger, in a time equal to seconds/ minutes from a single pulse. From this moment the tertiary effects of PEMF begin, of a purely biological type, which continue over a period ranging from a few hours to days and weeks starting from the first impulse and which include the transcriptional activation of various genes into the cell nucleus with the production of growth factors and other proteins and transmembrane receptors that will result in the orientation of the cells, regardless of the tissue they are part of, to regeneration and restore homeostasis [1,3,8,11-13] (Figure 1).
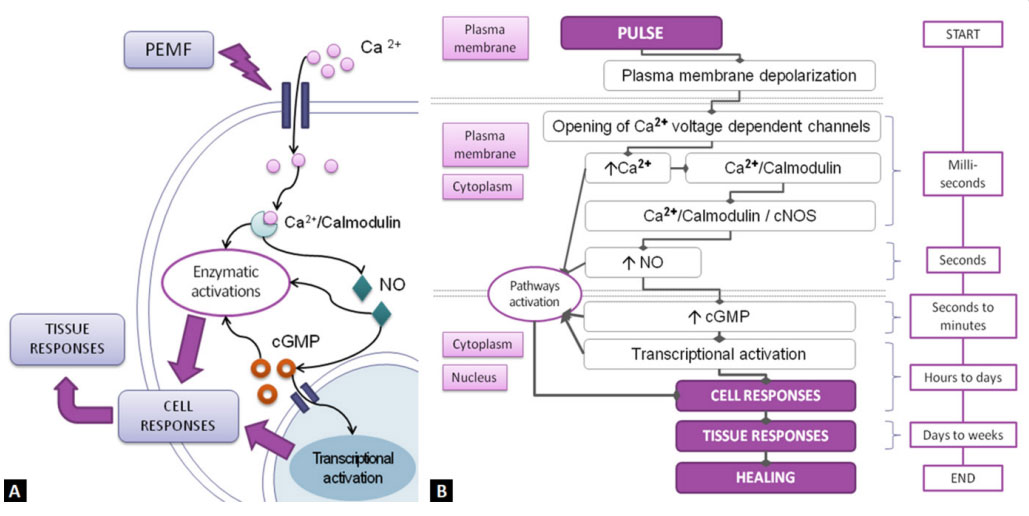 Figure 1: Mechanisms of action of PEMF on the cell. The most accredited current model sees the biochemical pathways activated by the calcium ion (Ca2+) and
subsequently by the nitric oxygen (NO) and cGMP as the key mechanisms of the action of PEMFs at the cellular level and, as a consequence, on the responses of
tissues. (A) General graphical representation of the model; (B) Schematic detail of the sequence of events starting from the first PEMF pulse with the alignment
of the temporal flow diagram of the individual events and the involvement of the various cellular compartments [3,7,8,11].
Figure 1: Mechanisms of action of PEMF on the cell. The most accredited current model sees the biochemical pathways activated by the calcium ion (Ca2+) and
subsequently by the nitric oxygen (NO) and cGMP as the key mechanisms of the action of PEMFs at the cellular level and, as a consequence, on the responses of
tissues. (A) General graphical representation of the model; (B) Schematic detail of the sequence of events starting from the first PEMF pulse with the alignment
of the temporal flow diagram of the individual events and the involvement of the various cellular compartments [3,7,8,11].What is observed macroscopically is the reduction of inflammation, pain, edema, and complete tissue regeneration, including neovascularization and remodeling of the extracellular matrix up to complete restoration of the injured tissue [2,5]. All the cells involved in an injury respond to the action of PEMF, including endothelial cells (which will rebuild the injured blood vessels), fibroblasts (which will proliferate and repair the injured extracellular matrix), muscle cells, chondrocytes, and osteoblasts (which will undergo a more rapid and efficient proliferation) [12]. The activity of the cells of the immune system, in particular the inflammatory component, is instead sedated (lowering of the levels of interleukins) [5] and the activation of monocytes to macrophages is favored to clean the injured area from microorganisms, foreign bodies, and dead cells. Ultimately, of the possible outcomes of acute inflammation, i.e. necrosis, chronic inflammation, and healing, PEMF blocks the first two and favors the regenerative outcomes of cells and tissues.
PEMF, as previously mentioned, promotes tissue healing and find application in various fields of medicine to promote healing following various kinds of traumas, injuries, post-surgical wounds, and inflammations. Pathologies that find the effective application of this technology include bone fractures, arthritis, osteoarthritis, acute and chronic inflammation, edema, pain, chronic pain, wounds, and chronic wounds [3,7,10,12]. PEMFs are also applied in physiotherapy, orthopedics, osteopathy, rehabilitation practice, sports medicine, and fitness, including the neuromuscular recovery of the post-workout athlete. The pathologies and disorders that may be included, in addition to those already listed above, concern all injuries, traumas, and inflammations affecting the shoulder, elbow, hand, knee, spine, hip joint, ankle joint, and foot [4,11,12,14]. Also included may be all pains of an arthritic nature, pain at the level of tendon insertions (e.g. tennis arm, golfer’s elbow, pitcher’s shoulder, and scapulohumeral periarthritis), overload syndrome,patellar pathology, meniscal pathology, degeneration of the intervertebral discs and vertebral joints, “witch stroke”, cervical spine pain due to “whiplash”, ischial pain, muscle contractures, and post-traumatic outcomes. All degenerative pains, painful tendon insertions, bursitis, Achilles tendon inflammation, calcaneal spine, and flat foot pain also may benefit [10,12,15]. Recent studies also regard their application as an adjuvant treatment for complex diseases such as cardiovascular diseases, diabetes, neurological disorders, microbial infections, and tumors [1,10,11]. PEMFs have no known side effects [3].
PEMF has its main and historical application in the treatment of wounds. Wound healing is a complex process involving cascades of inflammatory, proliferative, immune, and tissue remodeling reactions [16]. Clinical studies have shown that treatment with PEMF can promote and accelerate the healing of both fresh and recent wounds, including post-operative wounds, as well as chronic wounds such as pressure sores and diabetic leg and foot ulcers. The mechanism underlying this ability appears to be partly due to the increased vascularization induced in the tissue by stimulation via PEMF, but also to the better perfusion of the injured tissue and the better oxygenation, all important factors for wound repair [1,11,16,17].
PEMF stimulates endothelial cells to reproduce and rebuild injured blood vessels, increasing angiogenesis over time in the tissues affected by the wound [1,18]. PEMF also stimulates fibroblasts to rebuild the injured extracellular matrix, stimulates epithelial cells to reproduce, and restore lost tissue continuity. A key and very interesting aspect are that stimulation using PEMF synchronizes the reproduction activity of the cells invested by the electromagnetic field in a way that no cell species can privilege the others. This particular mechanism is still under study but the effects are visible as the healing of fresh wounds occurs when treated with PEMF, mainly by the first intention, or similar to the first intention, rather than by the second intention. This means that there is less development of keloids and disfiguring scars [3]. Also, PEMFs reduce inflammation and pain from the first impulse. This is significant in the management of all wounds but especially post-operative wounds [1].
Bone repair requires the cooperation of specific bone cell types: osteoblasts and osteoclasts. PEMFs have been shown to affect bone repair through several mechanisms including the stimulation of fibrocartilage calcification in the space between bone segments, the increased blood flow and wound healing as a result of effects on calcium ion channels, and increased bone formation rate by osteoblasts [1,4,7,10,19,20]. On the contrary, it is found that osteoclastic activity is reduced. Furthermore, the use of PEMF as a treatment for unconsolidated fractures has proven to be very effective [4].
PEMF stimulation has been shown to have clinical efficacy for the treatment of arthrosis and arthritis, including osteoarthritis and rheumatoid arthritis [1,7,9,10,12,21]. PEMF suppresses the inflammatory response [5] and is an adjunct in the management and treatment of pain.
PEMF can reduce pain and increase mobility right from the first applications even in patients who suffer from the disorder and do not respond or cannot take corticosteroid-based drug therapy [7,10,12].
Very recent studies concern the possible application of PEMF as an adjuvant in the non-surgical treatment of tumor pathologies, in particular solid tumors [22,23]. Research and evaluations are underway both in vitro and in vivo on animal models and in the clinic on case studies. The first results seem very encouraging as there is a decrease in the vitality of cancer cells, a reduction/delay in tumor angiogenesis, and a decrease in the growth rates of cancer cells. PEMF also promotes apoptosis and/or necrosis of tumor cells [10,24-26]. The biochemical mechanisms underlying what is observed are not yet clear but it is assumed that PEMF, while in a normal cell induces it to homeostasis, in a tumor cell probably reactivates metabolic and signaling pathways bypassed or silenced by the neoplastic transformation process such that the cell is so directed towards apoptosis or necrosis.
In clinical applications, it has been seen that there are specific frequencies (tumor‐specific frequencies) that have effects on cancer cells [27] while some frequencies do not affect metastases or tumor cells but only on the healing of lesions, in particular in the post-surgical after exeresis of a solid tumor with better and faster wound healing, the reduction of recovery times and elimination of infections and post-surgical scar formation [28,29]. Despite the positive results of the first studies, the application of PEMF in the oncology field is still under study and requires further investigation.
PEMFs can penetrate completely through all types of tissues, from the skin to bone, and are capable of inducing cellular and tissue responses, including transcriptional activation. PEMFs are cheap, very simple to use, non-invasive, non-thermal, have no known side effects, and can play an important role in the complementary treatment of various human diseases or trauma, included bone fractures, arthritis, osteoarthritis, acute inflammation, chronic inflammation, edema, pain, chronic pains, wounds, and chronic wounds. Although the mechanisms of action of PEMFs have yet to be fully elucidated, they find interesting applications in medicine and various medical specialties, including sports medicine, orthopedics, and physiotherapy, and currently, it is under study their application, and their possible benefits, in complex and chronic-degenerative diseases, including cancer.
In conclusion, PEMFs may play an important role in medicine as a complementary treatment for various human diseases and further studies will clarify their mechanisms of action and extend their application fields both in human and veterinary pathology.
Thanks to Mr. Simone Pratellesi and Mr. Valerio Pratellesi for all the technical assistance, information, and material provided, including many scientific studies and preliminary data, which have been used as a reference and idea to deepen and expand the background of this review.
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
