 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Gatien AG Lokossou* , Pierre B Adjevi1, Omar AK Maladé1, Julien Azonnakpo1, Lamine Baba-Moussa2
, Pierre B Adjevi1, Omar AK Maladé1, Julien Azonnakpo1, Lamine Baba-Moussa2
1Research Unit in Applied Microbiology and Pharmacology of Natural Substances, Polytechnic School of Abomey-Calavi, Department Human
Biology Engineering, University of Abomey-Calavi, Abomey-Calavi, Bénin
2Laboratory of Biology and Molecular Typing in Microbiology, Department of Biochemistry, Faculty of Science and Technology, University of
Abomey-Calavi, Abomey-Calavi, Bénin
Correspondence to: Gatien AG Lokossou, Research Unit in Applied Microbiology and Pharmacology of Natural Substances, Polytechnic School of Abomey-Calavi, Department Human Biology Engineering, University of Abomey-Calavi, Abomey-Calavi, Bénin
Received date: September 14, 2022; Accepted date: September 30, 2022; Published date: October 07, 2022
Citation: Lokossou GAG, Adjevi PB, Maladé OAK, et al. Maternal Breastfeeding Methods, Breastmilk-Derived Antibodies and Cells Concentrations and Their Impact on Infant Morbidity: Results from a Prospective Cohort in Southern Benin. J Med Res Surg. 2022; 3(5): 99-106. doi: 10.52916/jmrs224088
Copyright: ©2022 Lokossou GAG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Breastfeeding is associated with long-term well-being including low risks for infectious diseases and non-infectious diseases such as asthma, cancer, autoimmune diseases and obesity during childhood. However, very few studies assessed the real-impact of different methods of breastfeeding on infant morbidity in low-income countries such as Benin. Our study aimed to evaluate the dynamic change of immune components and the impact on infant morbidity among Beninese children population. Seventy-six children and their mothers were enrolled in colostrum, transitional and mature milk groups at the District Hospital of Comè in Bénin. Breast milk samples were collected one-time from mothers to assess total IgA, IgG and IgM and leukocytes by using spectrophotometry and level of microscopy, respectively. Mean or proportion comparisons were appreciated using a Mann-Whitney or Fisher’s exact tests, when appropriate. Forty (54.63%), six (7.89%) and thirty (39.47%) mother-infant pairs were enrolled in colostrum, transitional and mature milk groups respectively. The total number of leucocytes and antibodies were different in colostrum, transitional and mature milk. The prevalence of breastfeeding was 90.79% (n=69) in the population with 69.74% (n=53) of exclusive breastfed. Forty-five infants (84.91%) among exclusively breastfed infants were healthy whereas 4 (25%) among mixed breastfed infants and 4 (57.14%) among formula group were healthy (p=0.001). The total leucocytes count and IgA, IgG and IgM concentration decreases from colostrum through transition milk to mature milk. Our data showed a prevalence of exclusive breastfeeding which is associated to a positive clinical outcome on infant’s health. We have also confirmed decrease of antibodies and leucocytes during the maturation of breast milk.
Human breast milk, Breastfeeding, Infant health, Immunity, Antibodies
Human Breast Milk (HBM) is considered as the optimal food to provide the developmental nutrients needs for infants under 2 years as well as shape their immune system [1,2]. In addition, to provide nutrients (proteins, lipids, and carbohydrates), HBM provides immune and non-immune components, bioactive molecules (cytokines/chemokines, lipids, hormones, enzymes and antibodies) and microbiota favoring the breastfed infant protection against numerous diseases [1,3,4]. Moreover, the infant’s immune system matures by interacting with the environment including encountering pathogens, establishing adequate tolerance to the environment and the developing microbiota. [2,5–7]. The roles of the different breast milk components are far from being completely understood. These cells include immune cells (B and T lymphocytes, regulatory cells, monocytes/macrophages, neutrophils, natural killer cells and innate immune cells), stem cells, progenitor cells, lactocytes, and myoepithelial cells. Various antibodies are also found in HBM [1,8,9]. In the past decades, several studies on humans have shown that influencing factors such as geography, infant sex, or the number of pregnancies impact the heterogeneity and composition of HBM. Indeed, Holmlund et al. have shown that the maternal country of birth influences the pro-and anti-inflammatory contents of HBM, resulting in susceptibility or not to immune-mediated diseases such as allergy or necrotizing enterocolitis [10]. Despite this fact, too many children are malnourished and a high number of newborns and infants continue to die due to infectious diseases because of a lack of knowledge about the composition of HBM and how these components shape the infant’s immune system. It has been reported that through a process called microchimerism, maternal cells are transferred to infant intestinal mucosal tissue, and from there, enter their circulatory system to populate other infant immune tissues [11]. In Benin, very few studies on breast milk and its benefits are available. Our study could modify the national policies and put breast milk back at the heart of the diet of Beninese infants by extending maternal leave for this purpose or facilitating the breastfeeding in public and private workers environment. Herein, our study aimed to evaluate the dynamic change of immune components in human colostrum, transitional and mature milk and the impact of breastfeeding on infant morbidity.
From July to October 2018, we enrolled in a prospective study 40, 6 and 30 mother-infant pairs in colostrum, transitional and mature milk groups at the District Hospital of Comè in Bénin. Inclusion criteria were as follows: women whose infants were cared in pediatrics, women who gave birth in the maternity ward or who were transferred to the neonatology ward with their infants. Twins were excluded from the study. None of these women had any acute infection and complications during pregnancy and breastfeeding. This study was approved by the Institutional Review Board of the District Hospital of Comè, Bénin. Written consent was obtained from the mothers prior to the one-time sampling and information collection. Questionnaires were used to collect the mother-infant pairs’ demographic and clinical information. Mothers’ information included age, type of milk, and breastfeeding. Infant’s information included gender and age.
Colostrum was collected within 5 days after delivery, transitional milk 15 days after delivery and mature milk 35 days postpartum. After cleaning the nipples with cotton soaked in alcohol, the breast was pressed and the milk collected in a sterile tube. All samples were collected from one side of breast, and transferred on ice to laboratory where samples were immediately processed. Sera from infant’s peripheral blood samples (7 ml) were collected one-time at inclusion and were stored at -20ºC.
The white cell count of the different types of milk was done using the microscope (Olympus CX23, Olympus-life science, France) after 1/20 dilution with Lazarus liquid (Dutscher, France).
The total concentration of immunoglobulins A, G and M was determined using semi auto chemistry analyzer spectrophotometer (Rayto RT-9200, Shenzhen, China) after 1/10 dilution of milk and or colostrum and sera.
Differences in participants’ characteristics in colostrum group, transitional group and mature milk group were compared using Fisher exact and Mann–Whitney test, when appropriate. Differences of the means of leucocytes and antibodies at different stages of lactation were evaluated by Kruskal-Wallis test. Statistical analyses were performed using Prism 5 software (San Diego, CA). All hypotheses were two-sided, and p-values<0.05 were considered statistically significant.
A total of 40 (54.63%) colostrum, 6 (7.89%) transitional and 30 (39.47%) mature were collected in this study. As showed in Table 1, the mean age of mothers was 28.18 ± 5.55 years whereas their infants aged from 1 day to 2 years after birth. Table 1 outlines the characteristics of mothers-infants in each group. Fifty nine children (77.63%) were aged under 6 months whereas 17 (22.36%) were aged between 7 and 24 months. The prevalence of breastfeeding was 90.79 % (n=69) in the population with 69.74% (n=53) of exclusive breastfed, 21.05% (n=16) of mixed breastfeed and 9.21% (n=7) of formula fed.
|
Factors |
Colostrum (n=40) |
Transitional milk (n=6) |
Mature milk (n=30) |
P value |
|
|
Maternal characteristics |
|||||
|
Age (Years) |
26.95 (± 5.05) |
28.31 (± 5.72) |
29.80 (± 5.92) |
0.06 |
|
|
Mode of breastfeeding n (%) |
|||||
|
Exclusive |
30 (%) |
4(%) |
19 (%) |
- |
|
|
Mixed |
9 (%) |
1 (%) |
6 (%) |
- |
|
|
Formula |
1 (%) |
1 (%) |
5 (%) |
- |
|
|
Infantile characteristics |
|||||
|
Age (Months) |
|||||
|
0 to 6 |
40 |
6 |
13 |
- |
|
|
7 to 24 |
0 |
0 |
17 |
- |
|
|
Gender |
|||||
|
Male |
23 |
6 |
12 |
- |
|
|
Female |
17 |
0 |
18 |
- |
|
|
Exclusive breastfed under 6 months |
30 (75%) |
4 (66.66%) |
7 (23.33%) |
|
|
Figure 1 shows that depending on the phase and stage of lactation, a dynamic change in the proportion of white blood cells in colostrum (5.07 109/L), in transitional milk (2.64 109/L), and in mature milk (5.34 108/L) (P<0.0001).
As showed in Figure 2A, IgG level is higher in colostrum (1344.13 ± 122.57 mg/L) compared to transitional milk (1121.33 ± 114.21 mg/L) (P=0.0003) and mature milk (800.33 ± 191.84 mg/L) (P< 0.0001). In the same manner, IgA level in colostrum (4626.09 ± 438.83 mg/L) is higher than transitional milk (4114.84 ± 376.49 mg/L) (P=0.0036) and mature milk (2224.38 ± 680.19 mg/l) (P<0.0001) (Figure 2B). IgM was also higher in colostrum (1914.40 ± 440.89) than in transitional milk (1054.76 ± 426.18 mg/l) (P<0.0001) and in mature milk (509.54 ± 243.88 mg/l) (P<0.0001) (Figure 2C). HBM antibody concentrations decrease during the time and become stable when the milk is mature. Indeed, as shown in Figure 2D, IgA is most concentrated antibodies in human breast milk whether in colostrum or in transition milk or even in mature milk (Figure 2D). The influence of maternal age at delivery on immunoglobulin concentrations was also studied. No significant relationship could be observed for IgA and IgG concentration according to maternal age. However, a trend towards higher IgM concentration seems to occur after 20 years of age (Table 2).
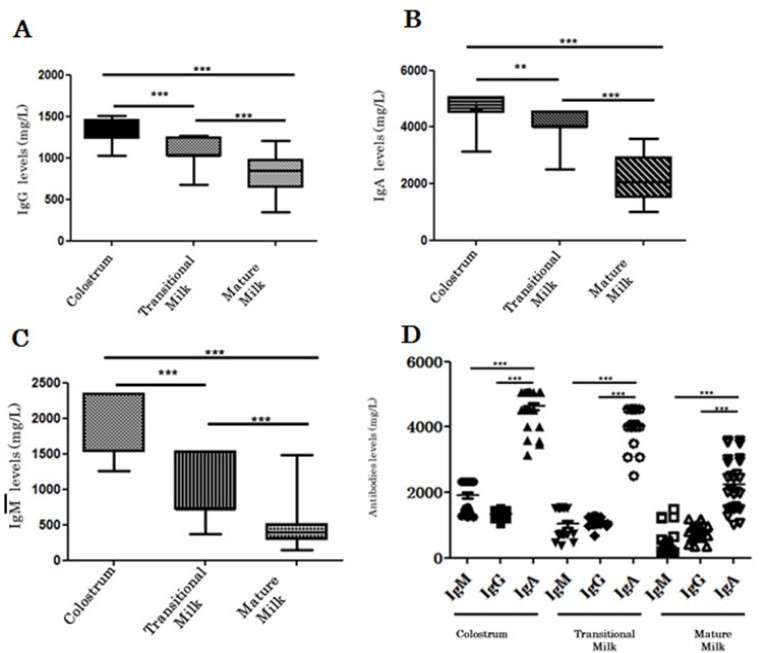 Figure 1: Change in IgA, IgG and IgM levels during human breast milk maturation.
IgG (A), IgA (B) and IgM (C) levels are higher in colostrum and decrease while human breast milk becomes mature. IgA is most concentrated antibodies in human breast milk whether in colostrum or in transition milk or even in mature milk (D).
Figure 1: Change in IgA, IgG and IgM levels during human breast milk maturation.
IgG (A), IgA (B) and IgM (C) levels are higher in colostrum and decrease while human breast milk becomes mature. IgA is most concentrated antibodies in human breast milk whether in colostrum or in transition milk or even in mature milk (D).|
Age (years) |
[10–20] |
[20–30] |
[30–40] |
P-value |
|
Parameters |
||||
|
IgA mg/L |
3068.09 ± 837.50 |
3809.29 ± 1080.97 |
3561.23 ± 975.46 |
0.22 |
|
IgG mg/L |
963.24 ± 165.18 |
1129.89 ± 231.91 |
1071.88 ± 236.03 |
0.148 |
|
IgM mg/L |
719.11± 464.78 |
1271.83 ± 647.09 |
1170.69 ± 618.88 |
0.08 |
In the exclusive breastfed group, 84.91% (n=45) of infants were healthy whereas 25% (n=4) and 57.14% (n=4) where healthy in the mixed breastfed and in the formula groups respectively (Table 3). In the exclusive breastfed group, 15.09% (n=8) infants suffered from different pathologies such as malaria, cold and cough, jaundice, bacterial infection or diarrhea (Table 5). In the mixed breastfed group, 75% of infants suffered from cold, jaundice, bacterial infection, diarrhea, abscess or respiratory distress (Table 5). Three formula fed infant suffered from respiratory distress or fever. Table 4 showed that the majority of sick children were aged less than 6 months (Table 4).
|
Type of breastfeeding |
Healthy children |
Sick children |
Total |
P value |
|
|
Exclusive breastfed % (n) |
84.91 (45) |
15.09 (8) |
100 (53) |
0.001 |
|
|
Mixed breastfed % (n) |
25 (4) |
75 (12) |
100 (16) |
||
|
Formula % (n) |
57.14 (4) |
42.86 (3) |
100 (7) |
||
|
Type of breastfeeding |
0 to 6 months |
7 to 24 months |
|
|
Exclusive breastfed |
(n=41) |
(n=12) |
|
|
Healthy children |
33 |
12 |
|
|
Sick children |
8 |
0 |
|
|
Mixed breastfed |
(n=14) |
(n=2) |
|
|
Healthy children |
4 |
0 |
|
|
Sick children |
10 |
2 |
|
|
Formula |
(n=4) |
(n=3) |
|
|
Healthy children |
2 |
2 |
|
|
Sick children |
2 |
1 |
|
Diseases |
Exclusive breastfed (n) |
Mixed breastfed (n) |
Formula (n) |
|
|
Abscess |
0 |
1 |
0 |
|
|
Bacterial infection |
1 |
1 |
0 |
|
|
Cold |
1 |
1 |
0 |
|
|
Diarrhea |
2 |
3 |
0 |
|
|
Fever |
0 |
0 |
1 |
|
|
Jaundice |
3 |
4 |
0 |
|
|
Malaria |
1 |
0 |
0 |
|
|
Respiratory infection |
0 |
2 |
2 |
Furthermore, in breast milk from mother who exclusively breastfed, the concentrations of IgA (3.903 ± 1.151 g/l), IgG (1.147 ± 2.639 g/l) and IgM (1.308 ± 0.747 g/l) were higher compared to milk from mothers who have given mixed breastfeeding (IgA=3.33 ± 1.16 g/l, IgG=1.15 ± 0.26 g/l and IgM=1.04 ± 0.63g/l) or those who have given formula to their infant (IgA=2.627 ± 1.228 g/l, IgG=0.892 ± 0.294 g/l and IgM=0.401 ± 0.047 g/l) (p=0.013, p=0.018, p=0.017 respectively) (Table 6).
| Type of breastfeeding | Exclusive breastfeeding |
Mixed breastfeeding |
Formula |
P value |
| IgA mg/L | 3903.34 ± 1151.93 |
3329.07 ± 1165.75 |
2627 ± 1228.2 |
0.013 |
| IgA mg/L | 1147.82 ± 263.97 |
1019.81 ± 293.11 |
892.71 ± 294.77 |
0.018 |
| IgA mg/L | 1308.90 ± 747.85 |
1036.42 ± 634.00 |
401.76 ± 46.89 |
0.017 |
Our observations showed that between 0 to 6 months, the mean of IgG levels were 3982.06 mg/L in healthy infants while it was slightly higher in infants who suffered from bacterial infection (4191.13 mg/L), jaundice (4197.41 mg/L), respiratory infection (5030.49 mg/L), digestive infection leading to diarrhea (4819.91 mg/L) or fever (4212.55 mg/L) (Figure 3). Peripheral blood IgM levels were relatively lower in children who had been ill compared to healthy children (Figure 3). Similarly, IgA levels were relatively lower in the peripheral blood of children who had been sick, except in children who had diarrhea where the IgA concentration was slightly higher (Figure 3).
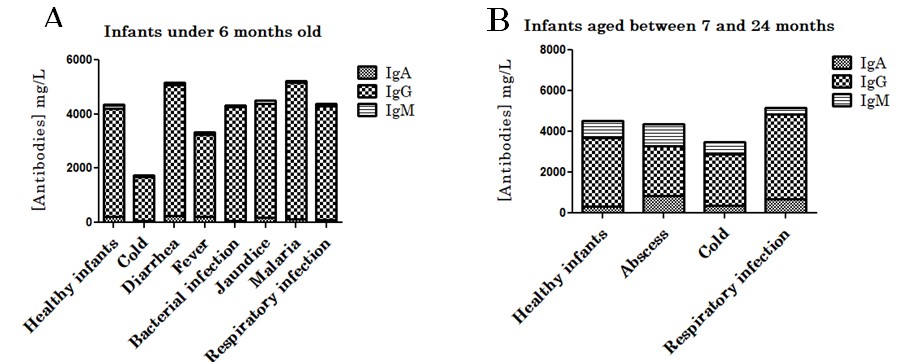 Figure 3: Peripheral blood IgA, IgG and IgM levels in healthy vs sick infants depending of age. Antibodies levels in infants under 6 months (A) and infants aged between 7 and 24 months (B).
Figure 3: Peripheral blood IgA, IgG and IgM levels in healthy vs sick infants depending of age. Antibodies levels in infants under 6 months (A) and infants aged between 7 and 24 months (B).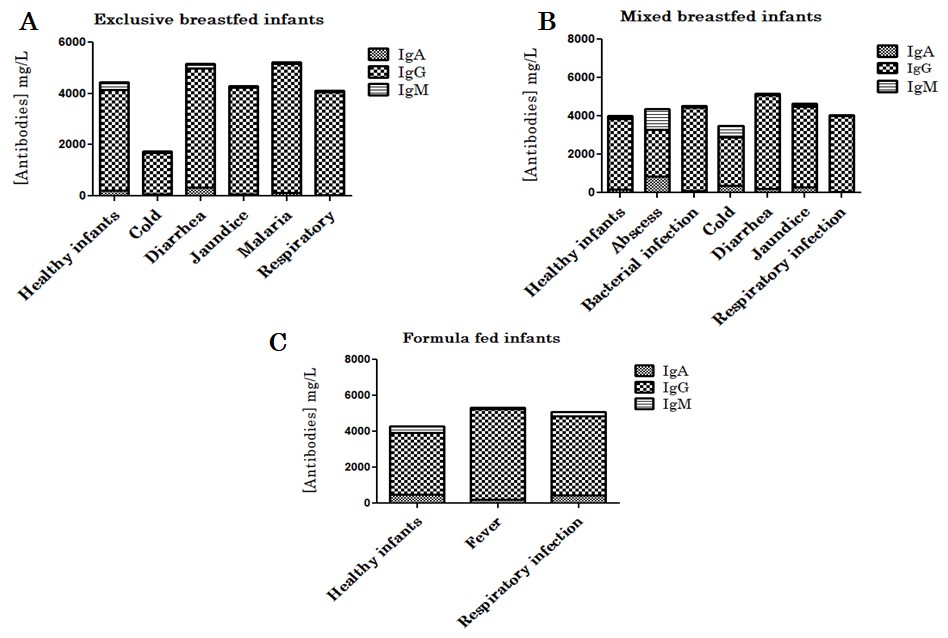 Figure 4: Peripheral blood IgA, IgG and IgM levels in healthy vs sick infants depending of the type of breastfeeding. Antibodies levels in exclusive breastfed (A), mixed breastfed (B) and formula fed (C) infants.
Figure 4: Peripheral blood IgA, IgG and IgM levels in healthy vs sick infants depending of the type of breastfeeding. Antibodies levels in exclusive breastfed (A), mixed breastfed (B) and formula fed (C) infants.Numerous immunological and cellular components are present in Human Breast Milk (HBM) and significantly affect the development of the newborn, its immune development and health [1]. Although the presence of these components has been well demonstrated, less is known about their specificity against microbes and childhood diseases [1,12]. Many factors, including race, ethnicity, environment, diets, culture, age etc might influence the composition of human breast milk [1]. In this study, we confirmed in a part of Beninese population the composition of colostrum, transitional and mature milk and the importance of exclusive breastfeeding in prevention against communicable and non-communicable disease. It was clearly demonstrated in this study that the total cell count and immunoglobulin concentration decreases from colostrum through transition milk to mature milk, as in previous observation [1,13-15]. Our data shown that HBM antibody concentrations decrease during the time and become stable when the milk is mature (Figure 2D) [15,16]. This observation confirms the importance of colostrum in the immune protection transmitted by the mother to the newborn [17,18]. Our major finding is the role of exclusive breastfeeding in protection against childhood disease which confirm the previous observations [19-21]. The influence of maternal age at delivery on immunoglobulin concentrations was also studied. No significant relationship could be observed for IgA and IgG concentration according to maternal age. However, a trend towards higher IgM concentration seems to occur after 20 years of age [22,23]. Although breast milk provides different immunoglobulin isotypes, IgA is more prominent and in the neonatal gut neutralizes bacterial and viral pathogens by binding to them. This neutralization of the microorganisms limits then their ability to interact and interfere with epithelial cells [1,24-27]. These characteristics easily explain the high concentrations of IgA found in human breast milk according to different types of lactating, especially in colostrum [1,22]. In addition, 90.79 % of the children in our study were breastfed. Children under-6 months were the most likely to be breastfed [28,29]. Among the breastfed children, 69.74 % had been exclusively breastfed. Among exclusive breastfed infants 77.36 % (n=41) were aged less than 6 months [1,28]. This confirms the natural tendency of mothers to breastfeed their newborns without necessarily understanding the immunological benefits and easily support the recommendation of the World Health Organization [2].
Depending on the type of breastfeeding, the concentration of immunoglobulins in breast milk varies considerably. Indeed, the concentration of IgA (2627 ± 1228.2 mg/L), IgG (892.71 ± 294.77 mg/L) and IgM (401.76 ± 46.89 mg/L) decreased in the milk of mothers who did not breastfeed their child compared to those who exclusively breastfed their child (IgA=3903.34 ± 1151.93 mg/L, IgG=1147.82 ± 263.97 mg/L and IgM=1308.90 ± 747.85 mg/L) or those who have given mixed breastfeeding (IgA=3329.07 ± 1165.75 mg/L, IgG=1019.81 ± 293.11mg/L and IgM=1036.42 ± 634.00 mg/L). Considering the amount of antibodies in maternal milk, the passive transfer of antibodies is very useful and depends on the previous and current exposure of the mothers to microorganisms [1,18,30]. Furthermore, our observations showed that children who were formula breastfed (42.85%) or mixed (75%) were more likely to be sick. Nowadays, it is clear that human breast milk and especially colostrum are protective against childhood infectious diseases and their recommendation must be strengthened [1,2,18,22]. Our study still has some limitations. Firstly, the number of the participants was too small and might skew the statistical analysis. More other, it would be better for this study to collect human colostrum, transition milk and mature milk from the same mother, which could better reflect the change in breast milk. Finally, more efforts are still needed to explore the specificity of breast milk-derived cells and antibodies and their effects on childhood disease. Peripheral blood IgM levels were relatively lower in children who had been ill but overall elevated IgG levels suggesting that these children have an established memory response or have benefited from transfer of IgG to peripheral blood after breastfeeding. Moreover, the levels of IgG in sick children are high and suggest an increase of IgG levels in the mammary glands of mother’s breastfeeding their child. Indeed, during breastfeeding, the infant’s mouth-derived pathogen may be retro-transferred via the nipple to breast and results in a mammary immune response [18,31,32]. These immune response leads to specific antibodies production in milk and fed to the infected infant [33-35]. Cleary, maternal, and infant infections stimulate a rapid leukocyte response in breast milk [18]. IgA levels were relatively lower in the peripheral blood of children who had been ill except in children who had diarrhea whose IgA concentration was slightly higher. This suggests a probable passage of IgA from the digestive mucous membranes into the blood, especially since it has been demonstrated that a sick child induces more antibody production by the breastfeeding mother [33-35].
The majority of infants cared at the district hospital of Comè in Bénin had been exclusively breastfed. The latter were much less likely to suffer from childhood diseases than those who had received mixed breastfeeding or formula. It is clear that the antibodies and cells transferred by the mothers during breastfeeding contributed significantly to this natural protection. It is also essential to assess the interference between maternally induced antibodies and cells following natural exposure to microorganism and vaccine antigens.
Gatien AG Lokossou and Pierre Bessan Adjévi conceived and designed the study. Material preparation, data collection and analysis were performed by Pierre Bessan Adjévi, Julien Azonnakpo and Gatien AG Lokossou. Gatien AG Lokossou conceived and wrote the manuscript. All authors commented, revised and edited the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was not funded.
The authors would acknowledge all women and their infants included in this study.
