 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Tsuneo Ishida*
2-3-6; Saido; Midori-Ku; Saitama-Shi; Saitama-Ken; ╤ 336-0907; Japan
Correspondence to: Tsuneo Ishida; 2-3-6; Saido; Midori-Ku; Saitama-Shi; Saitama-Ken; ╤ 336-0907; Japan.
Received date: August 26; 2021; Accepted date: September 6; 2021; Published date: September 13; 2021
Citation: Ishida T (2021) Glaucoma Progressing Stage Estimated from Visual Field Test Data with Glaucoma Patient; and Zinc(Ⅱ) Induced Regenerative Ativity from Optic Nerve Damage. J Med Res Surg 2(5): pp. 1-4. doi: 10.52916/jmrs214057
Copyright: ©2021 Ishida T. This is an open-access article distributed under the terms of the Creative Commons Attribution License; which permits unrestricted use; distribution and reproduction in any medium; provided the original author and source are credited.
Glaucoma progressing stages (Stages 1~5) estimated from Visual Field Index (VFI); Mean Deviation (MD); and Pattern Standard Deviation (PSD) data has been elucidated; in which the glaucomatous pathology is in the proceeding stage 2-3 with T. Ishida's glaucoma patient compared with referring with VFI; MD; and PSD values to the literatures. Zinc(II) induced VFI improvement should be taken into account when interpreting rates of VFI change over time that zinc promotes Retinal Ganglion Cells (RGCs) survival; in which zinc intake in RGCs survival may be zinc acetate 25-50mg/day. Zinc(II) induced recovery activity from optic nerve damage of the eye consists of four processes as follows. (1)Intraocular inflammatory stimulation process; Zinc concentrations 123-292μg/g inhibit intraocular inflammation with atypical growth factor oncomodulin (Ocm) binding to its cognate receptor on RGCs. (2) RGC survival process; Zn2+ chelators enhance RGC survival and promote axon regeneration through the optic nerve. (3) Neural axon regeneration process; Zn2+ chelation promotes axon regeneration. Norepinephrine Transporters (Net) inhibitor promotes RGCs survival and axonal regeneration. (4)Eye to brain pathway process; Zn2+ chelator TPEN promotes both enduring RGC survival and considerable axon regeneration. Zinc induced recovery for NO production in RGCs that the NO conveys from the eye to the brain through the axons of RGCs; in which zinc concentration 100 μM may be suited for the optic nerve recovery. Accordingly; Zinc(II) could enhance optic nerve damage recovery that Zn2+ may be bound with optic nerve damage proteins; in which Zn2+ ions may bind with intraocular protein; RGC survival protein; axonal protein; and optic nerve disorder proteins during recovery process by Zn2+ ions-centered tetrahedrally binding proteins molecular coordination pattern.
Keywords: Glaucoma Proceeding 5 Stages, VFI/MD/PSD Improvement, Zinc(II) Chelation, Intraocular Inflammatory Stimulation, RGC Survival, Axonal Regeneration, Eye-to-Brain
ACE2: Angiotensin Converting Enzyme 2; ACs: Amacrine Cells; AMD: Age-Related Macular Degeneration; COVID-19: Coronavirus Disease-19; CNS: Central Nervous System; COX-2: Cyclooxygenase-2; HDGF: Hepatoma-Derived Growth Factor; IOP: Intraocular Pressure; Klf9: Kruppel-Like Transcription Factor 9; MD: Mean Deviation; Net: Norepinephrine Transporters; NLR: Nod-Like Receptor; NLRP3: NLR Family, Pyrin Domain Containing 3; nNOS: Neuronal Nitric Oxide Synthase; NO: Nitric Oxide; NOS: NO Synthetase; Ocm: Oncomodulin; PDPP: Pattern Deviation Probability Plot; PSD: Pattern Standard Deviation; TPEN: C26H28N6; RGCs: Retinal Ganglion Cells; RPE: Retinal Pigment Epithelium; slc30a3: Solute Carrier Family 30 (Zinc Transporter) 3; TDPP: Total Deviation Probability Plot; TMPRSS2: Transmembrane Protease, Serine 2; TPEN: N,N,N’,N’-Tetrakis (2-Pyridylmethyl) Ethylenediamine; VF: Visual Field; VFI: Visual Field Index
COVID-19 virus pandemic continues to disrupt the delivery of ophthalmic care that more uncertainty of glaucoma care, thechange in glaucoma patient experience and attitudes, visual acuity deterioration as well as true glaucomatous progression caused by the COVID pandemic could elucidate the psychological and physiological impacts [1]. In the COVID-19 epidemic, coronaviruse may bind to corneal and conjunctival Angiotensin Converting Enzyme 2 (ACE2) receptors that serve as the key cell-surface receptor for SARS-CoV-2 that binds the viral spike protein, and transmembrane protease, serine 2 (TMPRSS2) is known to be an important cell surface-associated protease that allows viral entry following binding of the viral spike protein to ACE2 [2]. However, whether ocular surface cells possess these key factors for cellular susceptibility to viral infection remains unclear [3].
Glaucoma is a neuronal disease with susceptible optic nerve that reduced Intraocular Pressure (IOP) and protecting of Retinal Gang-lion Cells (RGCs), will always be an important treatment, but should be enhanced with other neuroprotective treatment [4]. Glaucoma has been treated as a disease of early cellular senescence that the existing evidence to support this view of glaucoma is examined in the light of glaucoma pathophysiology as a premature aging process, in which examined on longevity, retinal ganglion cell death, stem cells present opportunities and prevent repair optic nerve damage, and approaches to saving sight in glaucoma [5].
Index of the visual function of the eye called Visual Field Index(VFI) is the aggregate percentage of visual function for a given field at each point where the visual thresholds are estimated that VFI is calculated from the Pattern Deviation Probability Plot (PDPP) in eyes with a Mean Deviation (MD) better than 20 decibels (dB) and from the Total Deviation Probability Plot (TDPP) in eyes with a MD worse than 20 dB [6]. The VFI is intended for use in calculating rates of progression and staging glaucomatous functional damage and the central points have more weight than the peripheral that the VFI can range from 100% (normal visual field) to 0% (perimetrically blind field) [7]. The other, Pattern Standard Deviation (PSD) is known to increase in the initial stages of glaucoma as visual field damage worsens, generalized involvement of the visual field, and the PSD once again starts decreasing that PSD values increase as irregular depression of visual field sensitivity progresses and the values decrease as visual field damage progresses to the point of causing an overall reduction in sensitivity. Hence, PSD is considered to be an inappropriate parameter for determining the stage of glaucoma [8].
On the other hand, abundant trace transition metal element Ag(I), Cu(II), Zn(II) have an important role for anti-bacterial, anti-viral, and anti-cancerous activities in the human body [9- 11]. In the eye, zinc(II) could be useful for recovery of optic nerve damage injury that zinc is essential in the retina, choroid, and cornea that zinc interacts with taurine and vitamin A and modifies receptor plasma membranes, regulates the lightrhodopsin reaction, modulates synaptic transmission, and serves as an antioxidant.
Thus, Zn has glaucoma pathophysiology that zinc induced beneficial retinal metabolism, Age-related Macular Degeneration (AMD), vision recovery activity, and light-induced retinal injury are involved [12].
In this case report review, firstly, glaucoma proceeding estimated from VFI, MD, PSD data with glaucoma patient is analyzed, secondly, zinc(II) induced glaucoma VFI improvement is argued, and lastly, zinc(II) induced regenerative activity from optic nerve damage is discussed, subsequently the recovery molecular mechanism from zinc-binding optic nerve damage proteins is clarified
Glaucoma staging system based on octopus visual field (Stages 0 through 5) can have been classified from resulting of glaucoma visual field test that Stage 1–Early glaucoma, Stage2–Moderate glaucoma, Stage 3–Advanced glaucoma, Stage 4– Severe glaucoma, and Stage 5–End-stage glaucoma/blind are categorised [13].
Figure 1 shows visual test results that visual field tests for VFI, MD, and PSD have been obtained in left eye of T. Ishida’s glaucoma patient from 2016 to 2021 years (①~⑧) using the Humphrey field analyzer. Table 1 exhibits glaucoma staging system based on Humphrey visual field (Stages 0 through 5). Glaucoma proceeding stage 1~stage 5 estimated from data of VFI, MD, PSD values with glaucoma patient can be elucidated and judged, by which the respective values of VFI, MD, and PSD data are referred to VFI [14], MD [13], and PSD [14, 15] literatures. Hence, glaucoma stage proceeding with T. Ishida’s glaucoma patient may be judged to be estimated under the stage 2~3 proceeding at this time of 15/04/2021.
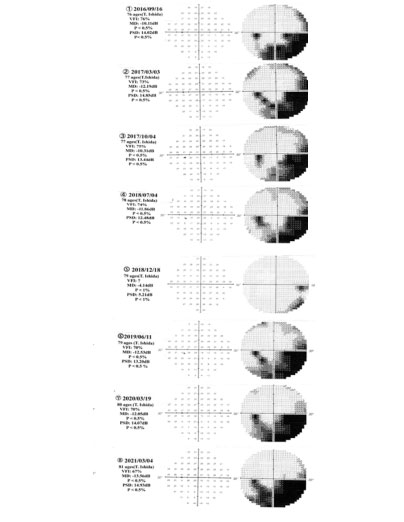 Figure1: Visual field test of VFI, MD, and PSD values obtained in left eye
of glaucoma patient (T. Ishida) from 2016 to 2021 years (①~⑧) using the
Humphrey field analyser.
.
Figure1: Visual field test of VFI, MD, and PSD values obtained in left eye
of glaucoma patient (T. Ishida) from 2016 to 2021 years (①~⑧) using the
Humphrey field analyser.
.Table 1:Glaucoma proceedings (Stage1~Stage5) estimated from Visual Field Index (VFI), Mean Deviation (MD), and Pattern Standard Deviation (PSD) data with T. Ishida’s glaucoma patient that has been referred to the VFI [14], MD [13], and PSD [14, 15] literatures.
| Stages VFI(%) | 9/16/2016 | 3/3/2017 | 10/4/2017 | 7/4/2018 | 12/18/2018 | 6/11/2019 3 | 3/19/2020 | 3/4/2021 |
| Stage 0; No defect stage | ? | |||||||
| Stage 1; (early) 82 <= VFI | PSD= 5,21dB |
| Stage 2 ;(moderate) 63 <= VFI <= 81 | VFI=76 MD= -10.11 dB | VFI=73 | VFI=75 MD=-10.31dBPSD=13.44dB | VFI=74 MD=-11.86dBPSD=12.48 dB | VFI=?PSD=5,21dB | VFI=70 PSD=13.20dB | VFI=70% | VFI=67 |
| Stage 3;(advanced) 43% <= VFI <= 62 % | PSD=14.02dB | MD= -12.19 dB PSD=14.85dB | MD=-12.53dB | MD=-12.05dB PSD=14. 07dB | MD=-13.56dB PSD=14.93dB | |||
| Stage 4; (severe) 23 % <= VFI <= 42 % | ||||||||
| Stage 5; (end) VFI <=22% | dB=decibel |
MD; Stage 0; >0.00, Stage 1; -0.01~-5.00, Stage 2; -5.01~-12.00, Stage 3; -12.01~-20.00, Stage 4; -20.01~or worse, Stage 5; No Humphrey Visual Field (HVF) in worst eyes PSD; Stage 0 ?, Stage 1; 2.10 to 13.57dB. Stage 2; 8.70 to 18.53dB, Stage 3; 12.34 to 19.14dB, Stage 4; 8.57 to 16.96dB, Stage 5; to 18.95dB.
Improvement of the VFI occurs with the reduction of Intraocular Pressure (IOP) to stabilize optic nerve and Visual Field (VF) deterioration [16]. Rates of progression can be evaluated through measuring structural and functional changes of the optic nerve. Very fast rates progression of MD progression of -1.5 dB/year are generally problematic and much slower rates also may be deleterious for particularly those diagnosed with late disease [17]. Zinc(II) induced VFI improvement may be involved that zinc promotes RGCs survival, hence, VFI improvement, considering that VFI substantially underestimates the amount of neural loss early in the disease and disease severity should be taken into account when interpreting rates of VFI change over time, in which zinc intake in RGCs survival may be zinc acetate 25-50 mg/day [18]. The role for zinc for the pathogenesis of AMD needs to be clarified in normal visual processing and its presumed involvement in the degeneration of the retina, as zinc predictably influences many intra- and extracellular functions. Thus, the final achievement is thought to be the retention of optimal zinc balance in the eye, which may slow the progression or even prevent the development of AMD [19]. However, the relationship between zinc induced improvement of VFI and glaucoma stage system reduction possesses difficult matters such as under the COVID-19 pandemics, whereupon these problems are remained unclear.
The optic nerve that consists of part of the Central Nervous System (CNS), has many obstacles limiting axonal regeneration will provide a rational basis that can be used to develop future approaches to optic nerve and axonal regeneration in the CNS that RGCs comprise the optic nerve axon, developmentally lose their intrinsic capacity to regenerate after injury [20]. Optic nerve regeneration is that normally suppress axon regeneration and the ability of retinal ganglion cells, the projection neurons of the retina, to survive after nerve injury that axon regeneration through the injured optic nerve [21]. Considering on treatment of acute optic neuropathy and end-stage disease, sustain RGC survival, axonal regrowth after injury, and robust axonal regeneration have been achieved with combinatorial approaches, involving gene deletion, pharmacological approaches, and visual stimulation [22]. Recovery process including with inflammatory stimulation, exogenous neurotrophic factors, inhibiting cell-intrinsic suppressors, activation of the intracellular signaling pathway, and NO production (ROS blocking) is very important that RGC survival, axon regeneration, RGC death, and enduring RGC survival should be treated [23].
Thus, optic nerve recovery process may be shown that the process chiefly consists of intraocular inflammatory stimultion, RGC surviral and attenuate RGC death, neural axon regeneration, and eye-to-brain pathway of combination with correct reinnervation of regenerating axons to their appropriate targets in the brain.
Hence, zinc induced recovery activity from optic nerve damage of the eye is thought to possess four processes as follows.
Intraocular inflammation induces substantial levels of optic nerve regeneration and this effect is mediated in large part by the atypical growth factor Oncomodulin (Ocm), which binds to its cognate receptor on RGCs [24]. Zinc concentration in inhibition of intraocular inflammation is that the highest amount of zinc is concentrated in the Retinal Pigment Epithelium (RPE) (RPEchoroid, 292 ± 98.5 µg/g dry tissue), followed by the retina (123 ± 62.2 µg/g dry tissue) [25]. Thus, zinc inhibits oxidative damage and Nod-Like Receptor (NLR) family, pyrin domain containing 3 (NLRP3) inflammation, hence, zinc has a protective effect on spinal cord injury [26].
Zinc has been implicated as a critical mediator of neuronal injury that Zn2+ is a major suppressor of the regenerative potential of axons after nerve injury as well as a cause of neuronal death and Zn2+ enhances RGC survival and promotes axon regeneration through the optic nerve [27]. Zinc ions couldregenerate optic nerve damage proteins of injured RGCs that many of the proteins revealed in RGC survival and axonal regeneration proteins are novel potential targets for therapeutic interventions directed at preventing RGC degeneration and visual impairment and Hepatoma-Derived Growth Factor (HDGF) is a potent neuroprotective factor for injured adult RGCs [28]. Zn2+ chelation with chelators continues to enhance outcome after nerve damage, however, chelating Zn2+ results in both enduring RGC survival and considerable axon regeneration in amacrine cell processes after the optic nerve to be injured that Zn2+ chelators can augment axon regeneration after other types of CNS injury or protect RGCs in neurodegenerative diseases such as glaucoma [29]. The mobile Zn2+ increased rapidly in mouse retina after OH, leading to RGCs injury that Zn2+ chelators significantly enhanced the survival of RGCs and apoptosis mediated by mitochondrial disfunction could be the mechanism underlying Zn2+ toxicity [30].
The zinc chelation and zinc transporter ZnT-3 (encoded by gene slc30a3) also enhance RGC survival and regeneration [23], however, the elimination of vesicular Zn2+ transporter ZnT-3 promotes RGC survival and axon regeneration [29].
Promoting axon regeneration after CNS injury is becoming increasingly more accepted for zinc chelation therapy that provides strong neuroprotection while continuing to develop means to enhance axon regeneration further. Zn2+ chelation with Kruppel-Like Transcription Factor 9 (Klf9) suppression has promoting axon regeneration after optic nerve injury and may also be effective for treating other CNS injuries and diseases [24]. Chelate Zn2+ enhances retinal ganglion cell survival and promotes axon regeneration through the optic nerve, in which the mechanisms by which Zn2+ regulates cell survival and axon regeneration, with the ultimate goal of developing new ways to enhance recovery in patients with CNS damage [31].
As the first step of the eye-to-brain reconnection, long-distance axon regeneration is crucial in the restoration of visual function following optic nerve injury [23]. RGC/axon and brain neural regeneration may affect the outcome of the programmed cells that the methods after promoting RGC regeneration in the retina may facilitate the regeneration of other types of neurons in the brain and vice versa [32].
Zinc(II) assists optic nerve recovery that Zn2+ ions may promote axon and RGCs regeneration in eye-to-brain pathway [33]. Zinc concentration for recovery from optic nerve damage is that Zn2+ chelation (specific Zn2+ chelators TPEN [20–500 μM) promotes axon regeneration that the specific Zn2+ chelator TPEN (100 μM) (N,N,N’,N’-Tetrakis(2-Pyridylmethyl Ethylenediamine) is indicated appropriately [29]. Daily administration of 50 mg zinc sulphate can inhibit complement catabolism in AMD patients with increased complement activation. This could explain part of the mechanism by which zinc slows AMD progression [34]. Accumulation of zinc ions using zinc-chelator causes to retinal degeneration and Cyclooxygenase-2 (COX-2) formation that over accumulation of Zn2+ may be an indicator of degeneration of retinal neurons in abnormal conditions [35]. Further, zinc induced recovery for Nitric Oxide (NO) production in RGCs is that NO is synthetized by NO Synthase (NOS) and production of NO in Amacrine Cells (ACs) after optic nerve injury to the existence of RGC axon injury and NOS1 activation. NO can be efficient in RGCs degeneration that in glaucoma patients it may produce high amounts of NO causing neurotoxicity to the axons of RGCs [36]. The Nitric Oxide Synthase (nNOS) in RGCs should be provided that not excessive NO production may damage RGCs, but also a clue to the somatic events of glaucomatous optic neuropathy [37].
Table 2 represents zinc(II) ions-induced optic nerve regenerative activity of intraocular inflammatory stimulation, RGC surviral, neural axon regeneration, and eye to brain pathway during recovery process from optic nerve damage, and the recovery overall reaction of Zn2+ ions-binding with optic nerve proteins. Accordingly, the zinc ions-binding molecular mechanism is clarified as follows. Zinc(II) could control with optic nerve damage proteins that Zn2+ tends to bind in tetrahedral coordination modes, although there is other geometries such as octahedral and square planar [38] and that Zn2+ ions may be bound by Zn2+ ions-centered coordinated tetrahedrally with intraocular inflammatory protein, RGC survival protein, axonal regenerative protein, and optic nerve disorder proteins, respectively, during recovery process [39].
Table 2 Zinc ions-induced optic nerve regenerative activity from optic nerve damage during recovery process.
| Zn2+ ions | Zinc ions-induced optic nerve regenerative activity from optic nerve damage during intraocular inflammation, RGC survival, axonal regeneration, and eye-to-brain process | |||
| Intraocular inflam-matory stimulation | RGC survival | Neural axonal regeneration | Eye to brain pathway | |
→ Zn2+ • Zinc inhibits oxida- tive damage and inflammation in nerve cells. • Atypical growth factor oncomodulin (Ocm) binds to its cognate receptor on RGCs • Zinc concentration 123-292 μg/g inhibits intraocular inflammation |
→ Zn2+ • PTEN deletion, Klf-9 suppression, upregu- lation of osteopontin on RGC survival and regeneration • Mobile Zn2+ promote for promoting RGC survival• Zinc transporter ZnT-3 (encoded by gene slc30a3) knockout enhanced RGC survi- val and regeneration. • Elimination of vesi- cular Zn2+ promotes RGC survival and axon regeneration |
→ Zn2+ • Effective for CNS injury • Zn2+ chelation in combination with Klf9 suppression for promoting axon regeneration • Zn2+ chelationpromotes axon regeneration. • Norepinephrine transporters (Net) inhibitor promotes RGCs survival and axonal regeneration |
→ Zn2+ • Zn2+ chelator TPEN (100 μM) for recovery from optic nerve damage • 50 mg zinc sulphate can inhibit comple- ment catabolism in AMD patients • NO generation effect • NO lies upstream of Zn2+ elevation in ama- crine cell processes • NO conveys from the eye to the brain thro- ugh the axons of RGCs • Zinc concentration 100 μM for optic nerve recovery
|
|
| Zinc(II) induced optic nerve recovery molecular overall reaction;Zn2+ ions-binding with optic nerve disorder proteins may be regenerated by forming Zn2+ ions-centered coordinated tetrahedrally with optic nerve damage proteins such as intraocular inflammatory stimulation protein, RGC survival protein, neural axonal protein, and optic nerve disorder proteins. | ||||
Glaucoma progressing stages estimated from VFI, MD, PSD data with glaucoma patient is analyzed, and zinc induced glaucoma VFI improvement and enhanced regenerative activity from optic nerve damage during recovery process are clarified under the COVID-19 infectious pandemic.
Glaucoma staging system based on octopus visual field (Stages 0 through 5) can have been classified from resulting of glaucoma visual field test that Stage 1–Early glaucoma, Stage 2–Moderate glaucoma, Stage 3–Advanced glaucoma, Stage 4– Severe glaucoma, and Stage 5–End-stage glaucoma/blind are categorised that Glaucoma proceedings (Stage 1~ Stage 5) estimated from Visual Field Index (VFI), Mean Deviation (MD), and Pattern Standard Deviation (PSD) data has been elucidated and the glaucomatous pathology has been in the proceeding stage 2-3 with T. Ishida’s glaucoma patient, compared by referring to VFI, MD, and PSD literatures.
Zinc(II) induced VFI improvement may be involved that zinc promotes RGCs survival, VFI improvement, considering that VFI substantially underestimates the amount of neural loss early in the disease and disease severity should be taken into account when interpreting rates of VFI change over time, Zinc intake in RGCs survival may be zinc acetate 25~50 mg/day.
Zinc(II) induced recovery activity from optic nerve damage of the eye possesses four processes as follows.
In intraocular inflammatory stimulation process; Zinc concentration 123-292 μg/g inhibits intraocular inflammation with atypical growth factor oncomodulin (Ocm) binding to its cognate receptor on RGCs.
In RGC survival process; Zn2+ chelators enhance the survival of RGCs and apoptosis mediated by mitochondrial disfunction could be the mechanism underlying Zn2+ toxicity and the zinc transporter ZnT-3 (encoded by gene slc30a3) enhances RGC survival and regeneration.
In neural axon regeneration process; Zn2+ chelation promotes axonal regeneration. Norepinephrine transporters (Net) inhibitor promotes RGCs survival and axonal regeneration. In eye-to-brain pathway process; The accumulation of zinc ions using zinc-chelator and specific Zn2+ chelator TPEN promotes both enduring RGC survival and considerable axon regeneration. NO lies upstream of Zn2+ elevation in amacrine cell processes.
NO conveys from the eye to the brain through the axons of RGCs. The zinc concentration is 100 μM or 50 mg/day for the optic nerve recovery.
Accordingly, zinc(II) controls optic nerve damage proteins that Zn2+ ions may be bound with intraocular protein, RGC survival protein, axonal regenerative protein, and optic nerve disorder protein, respectively, during recovery process by Zn2+ ionscentered coordinated terahedrally binding proteins molecular mechanism.
The author declares there is no conflicts of interest.
None, author’s own expenses.
The author (Anti-bacterial, anti-viral, anti-cancer researcher by Ag+ , Cu2+. Zn2+ ions solutions) thanks Saitama City Hospital, Eye Doctor Maya Kimura for providing VFI, MD, PSD data with T. Ishida’s glaucoma patient.
