 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Correspondence to: Ricardo Hsieh, General Pathology of Department of Stomatology, School of Dentistry, University of São Paulo, Brazil; E-mail: r.hsieh@usp.br
Received date: March 5, 2020; Accepted date: March 12, 2020; Published date: March 20, 2020
Citation: Hsieh R, Lourenço SV (2020) Does BCL-2 Play Role in the Pathogenesis of Primary Oral Mucosal Melanoma?. J Med Res Surg. 1(2): pp. 1-3.
Copyright: © 2020 Hsieh R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Primary Oral Mucosal Melanoma represents 0.2 to 8% of all melanomas and 0.5% of all oral malignant neoplasia. The etiology still unknown, however, it has been suggested that head and neck mucosal melanomas change their genetic and metabolic pathways through intracellular cascades, which are associated with its etiopathogenesis mechanisms. The BCL2 protein is an integral part of the cell membrane, and it is also found in the cell nucleus, mitochondria, and endoplasmic reticulum. It is overexpressed in several malignant neoplasms, including cutaneouse ocular melanomas.
Among all evaluated cases, we found positive immunostaining of BCL-2 in 26/34 (76.47%) and they had a membrane and cytoplasmic pattern, and the intensity was variable. According to our results and the findings of the literature, it can be suggested that BCL-2 has an important role in melanoma pathogenesis, including Primary Oral Mucosal Melanoma and also melanoma metastases. It seems that BCL-2 could be an adjunct marker for POMM and also a target for treatment development. New researches involving BCL-2 and a larger primary oral mucosal melanoma cohort could corroborate the present study
Primary Oral Mucosal Melanoma (POMM), Tissue, Malignant neoplasm, Tumor cells
Primary Oral Mucosal Melanoma (POMM) is a very uncommon and fatal malignancy, which originated from malignant transformation and clonal expansion of neural crest-derived melanocytes found either in the basal cell layer of the oral epithelium or in the lamina propria of the oral mucosa (1-3). POMM represents 0.2 to 8% of all melanomas and 0.5% of all oral malignant neoplasia. It is associated with aggressive behavior, vertical growth pattern and high risk of metastasis (including lymph nodes, lung, bone, liver, and brain) [1,2].
The etiology still unknown, however, it has been suggested that head and neck mucosal melanomas change their genetic and metabolic pathways through intracellular cascades, which are associated with its etiopathogenesis mechanisms [3,4]. The BCL2 oncogene is located on chromosome segment 18q21.3, in a telomere-centromere orientation, encoding a family of antiapoptotic proteins that prolong cell survival. The BCL2 protein is an integral part of the cell membrane, and it is also found in the cell nucleus, mitochondria, and endoplasmic reticulum. It is overexpressed in several malignant neoplasms, including cutaneous ocular melanomas [5,6].
Due to the lack of studies involving the pathogenesis of POMM, the present study evaluated the expression of BCL-2 protein by Immunohistochemistry (IHC) of 35 Formalin-Fixed ParaffinEmbedded (FFPE) cases of Primary Oral Mucosal Melanoma organized in Tissue Microarray (TMA) from AC Camargo Cancer Center and University of São Paulo.
Among all evaluated cases, we found positive immunostaining of BCL-2 in 26/34 (76.47%), and they had a membrane and cytoplasmic pattern, and the intensity was variable. We only consider positive expression for BCL-2 proteins, when more than 25% of tumor cells were stained (Figure 1).
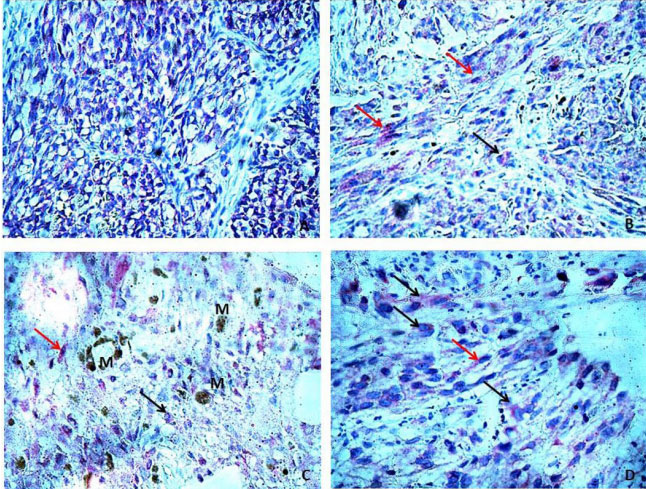 Figure 1: (A) Cytoplasmic expression of BCL-2 in polymorphous type melanocytes of POMM; (B) Red arrow: Cytoplasmic expression of
BCL-2 in spindle melanocytes of POMM, Black arrow: Cytoplasmic expression of BCL-2 in epithelioid melanocytes of POMM; (C) Red
arrow: Cytoplasmic expression of BCL-2 in spindle melanocytes of POMM, Black arrow: Cytoplasmic expression of BCL-2 in epithelioid
melanocytes of POMM, M: melanin; (D) Red arrow: Cytoplasmic expression of BCL-2 in spindle melanocytes of POMM, Black arrow:
Cytoplasmic expression of BCL-2 in epithelioid melanocytes of POMM.
Figure 1: (A) Cytoplasmic expression of BCL-2 in polymorphous type melanocytes of POMM; (B) Red arrow: Cytoplasmic expression of
BCL-2 in spindle melanocytes of POMM, Black arrow: Cytoplasmic expression of BCL-2 in epithelioid melanocytes of POMM; (C) Red
arrow: Cytoplasmic expression of BCL-2 in spindle melanocytes of POMM, Black arrow: Cytoplasmic expression of BCL-2 in epithelioid
melanocytes of POMM, M: melanin; (D) Red arrow: Cytoplasmic expression of BCL-2 in spindle melanocytes of POMM, Black arrow:
Cytoplasmic expression of BCL-2 in epithelioid melanocytes of POMM.The present study contemplated a very rare casuistry of Primary Oral Mucosal Melanoma. Our results showed most of the cases (76.47%) had a positive expression for BCL-2. There are few studies in the literature involving expression of BCL-2 protein and pigment disorders, moreover, the findings remain controversial. In 1994, van den Oord et al. observed that most of the benign pigment cell lesions and malignant pigment cell lesions were positive for BCL-2 and in cutaneous and lymph node metastasis showed negative or weak and focal expression [7]. Later, Tron et al. [8] showed that benign melanocytes from 3 of 4 normal skin biopsies and 5 of 7 common acquired nevi strongly expressed BCL-2, on the other hand, only 3 of 23 primary cutaneous melanoma and 3 of 9 metastatic melanoma showed staining [8]. Grossman et al. [9] reported that the apoptosis inhibitor BCL-2 was expressed in 26 of 30 (87%) malignant melanoma lesions, however at a reduced intensity compared with that of adjacent reactive lymphocytes [9].
In XXI century, Zhuang et al. (2007) observed that BCL-2 was expressed in 100% of benign nevi and thin melanoma (< 1.0mm) but was less in thick melanoma (>1.0 mm) (88%), subcutaneous (62%) and lymph node metastases (35%) [10]. A historical cohort of melanoma metastases showed that BCL-2 expression was detected in 74.3, 85.7, 82.4% of lymph node, subcutaneous and visceral metastases, respectively (5). In 2012, Prasad et al. 76 cases mucosal melanoma, including initial mucosal tumors, mucosal recurrences, and nodal/distant metastases, found in 74% of all evaluated cases (6). Hsieh et al. [11] found BCL-2 positive expression in 27/29 mucosal melanoma cases. Recently, Jurmeister et al. [12], observed BCL-2 positive expression in 45.4% of primary malignant melanoma.
According to our results and the findings of the literature, it can be suggested that BCL-2 has an important role in melanoma pathogenesis, including Primary Oral Mucosal Melanoma and also melanoma metastases. It seems that BCL-2 could be an adjunct marker for POMM and also a target for treatment development. Further researches involving BCL-2 and a larger POMM cohort could corroborate the present study
The authors declare no financial or non- financial conflicts of interest concerning the research, authorship, and publication of this article.
