 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Anum Sultan1* , Syeda Zehra Rizvi2, Dania Cioni3, Emanuele Neri4
, Syeda Zehra Rizvi2, Dania Cioni3, Emanuele Neri4
1Consultant Radiologist, Dr. Ziauddin hospital, Karachi, Pakistan.
2Resident Radiology, Dr. Ziauddin hospital, Karachi, Pakistan.
3Academic Radiology, University of Pisa, Italy.
4Chair Academic Radiology, University of Pisa, Italy.
Correspondence to: Anum Sultan, Consultant Radiologist, Dr. Ziauddin hospital, Karachi, Pakistan.
Received date: May 24, 2024; Accepted date: June 06, 2024; Published date: June 13, 2024
Citation: Sultan A, Rizvi SZ, Cioni D, et al. Diagnostic Accuracy of Combined Mammography and Ultrasound in the Detection of Malignant Breast Lesions Using Bi-RADS Classification Taking Histopathology as the Gold Standard. J Med Res Surg. 2024;5(3):55-62. doi: 10.52916/jmrs244137
Copyright: ©2024 Sultan A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Introduction: Breast cancer is the leading cause of cancer in women with an estimated 2.3 million new cases worldwide and a high mortality rate. The incidence of breast cancer has been increasing worldwide in the past few years with a similar trend of escalation in Pakistan. The age-standardized incidence rate of breast cancer in Pakistan is 104 per one million and the mortality rate is 65 per one million population. Limited studies have been done to evaluate the diagnostic accuracy of combined mammography and ultrasound with BI-RADS scoring in the detection of breast cancer and positive predictive value of its morphological descriptors in Pakistan.
Objective: Our study aims to determine the diagnostic accuracy of combined mammography and ultrasound in the detection of malignant breast lesions using BI-RADS classification taking histopathology as the gold standard and positive predictive value of its morphological descriptors.
Materials and Methods: This was a retrospective, cross-sectional analysis. All the patients presented with breast-related symptoms and for screening in whom mammography with complimentary ultrasound was performed were included. Mammography protocol includes image acquisition in craniocaudal and mediolateral oblique views. On ultrasound, all quadrants of the breast, retroaerolar region, and axilla were assessed. Patient stratification was done based on the age, clinical symptoms, and positive malignant lesions on histopathology; and frequency and percentage were calculated. Sensitivity, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and diagnostic accuracy was calculated. PPV of individual morphological descriptors were also calculated. The association of various morphological descriptors with malignancy was analyzed using a chi-square chart. p-value of less than 0.05 was considered significant.
Results: In 69 patients with suspicious imaging findings, 89.9% patients presented with breast lumps, 34.8% patients had pain, and 11.6% patients had nipple discharge. 8.7% had nipple retraction and 10.1% had skin changes. 52.2 % patients were post-menopausal and 46.1% patients were premenopausal. On histopathology, 88.4% patients had malignant disease and 11.5% were having benign lesions. The mean age of patients with malignant masses was 50.9 years+13.1 SD. No significant statical difference is noted between younger and older groups. The mean size of the malignant mass was 3.0 cm+1.8 SD. The sensitivity of combined mammogram and ultrasound was calculated to be 98.3%, specificity was 25.0%, PPV was 90.9%, NPV was 66.6% and diagnostic accuracy was 89.9%.
Conclusion: We conclude that the combined mammography and ultrasound serve as an important diagnostic tool, both for screening purpose as well as in patients with breast related symptoms for the diagnosis of breast cancer. Moreover, the morphological descriptors of malignancy on mammography and ultrasound as described by BI-RADS lexicon are reliable indicators of malignancy in patients with breast lesions.
Ultrasound, Mammography, BI-RADS classification, Breast cancer, Malignancy, Histopathology.
Breast cancer is the leading cause of cancer in women with an estimated 2.3 million new cases (11.7%) worldwide [1] and a high mortality rate [2]. According to GLOBOCAN 2020 report, the age-standardized incidence of breast cancer is 47.8 per one million with more than half a million deaths globally every year [2,3]. The incidence of breast cancer has been increasing worldwide in the past few years with a similar trend of escalation in Pakistan. The age-standardized incidence rate of breast cancer in Pakistan is 104 per one million and the mortality rate is 65 per one million population [1,2].
Over the past few decades, various advancements were made in the field of medicine for the early detection of breast cancer including clinical examinations, self-assessment, and screening programs as well as the use of multimodality imaging including mammography, ultrasound, and magnetic resonance imaging [3]. Being non-invasive, widely available, and cost-effective techniques, ultrasound and mammography are the primary imaging modalities used as baseline investigations for the assessment of breast tissue in women presenting with breast-related symptoms [3]. These are not only effective in the detection of breast lesions but are quite capable of characterizing them as benign and malignant [4].
First introduced in 1993, the American College of Radiology devised the BI-RADS lexicon of descriptors to homogenize the recording and documentation of mammographic findings in order to reduce the variations and discrepancies in mammography reporting, among radiologists in different hospitals thus aiding the communication between radiologists and other physicians [5,6]. Further editions were later published in 1995, 1998, 2003, and 2013 with the inclusion of descriptors of ultrasound and magnetic resonance imaging as well as shear wave elastography [6,7].
BI-RADS lexicon categorizes the breast findings into seven categories ranging from 0 to 6. Category 0 is labeled as inconclusive and needs additional imaging, category 1 as normal, category 2 as benign, category 3 as probably benign, category 4 as probably malignant, category 5 as malignant, and category 6 as biopsy-proven malignant [2,8,9]. Each category directs the physicians towards different management plans and accurate implementation of available treatment options. Category 0 demands additional imaging, and category 1 and 2 need routine annual screening. For category 3 lesions, 6 months follow-up assessment is recommended, category 4 and 5 lesion needs biopsy and histopathological evaluation and category 6 lesions require surgical excision when clinically appropriate [9].
Limited studies have been done to evaluate the diagnostic accuracy of combined mammography and ultrasound with BI-RADS scoring in the detection of breast cancer and its morphological descriptors in Pakistan. Our study aims to determine the diagnostic accuracy of combined mammography and ultrasound in the detection of malignant breast lesions using BI-RADS classification taking histopathology as the gold standard.
This was a retrospective, cross-sectional analysis conducted at the Dr. Ziauddin Hospital, Karachi from 1st January 2023 to 30th June 2023. All the patients presented with breast-related symptoms and for screening in whom mammography was performed were included. Complimentary ultrasound of all the patients was also performed. Patient demographics including age, gender, menopausal status, family history of breast cancer, and their presenting complaints were recorded. Patients having incomplete clinical information, imaging, or histopathological findings were excluded. Patients with BI-RADS category 0 in whom ultrasound was not performed were excluded.
Mammography was performed on a 2D digital analog mammography unit Lilyum, Metaltronica, Italy. Mammography protocol includes image acquisition in craniocaudal and mediolateral oblique views. Cone compression views were also obtained in a few patients to discriminate between normal breast parenchyma from breast lesions at the radiologist's discretion. Mammographic features of breast lesions including breast density, involved quadrant of the breast, mass shape, margins, presence of microcalcifications, skin thickening, architectural distortion, and intra-mammary lymph nodes were assessed. Ultrasound was performed on Toshiba Xario 200, Japan using a 10MHz high-frequency probe. All quadrants of the breast, retroaerolar region, and axilla were assessed. On ultrasound, lesions were evaluated for echogenicity, size, margins, vascularity, and presence of axillary lymphadenopathy.
Data collection was done by reviewing the patient's imaging and medical record. Images were interpreted by the radiologist having 5 years of experience and blinded to histopathological findings. BI-RADS category was assigned after each scan from 1 to 5. BI-RADS categories 1, 2, and 3 were considered negative, while BI-RADS categories 4 and 5 were considered positive. Histopathological findings were labeled as benign or malignant.
Patient data were analyzed using Statistical Package for the Social Sciences (SPSS), Version 20 (IBM Corp., Armonk, NY). Patient stratification was done based on the age, clinical symptoms, and positive malignant lesions on histopathology; and frequency and percentage were calculated. Sensitivity, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and diagnostic accuracy of combined mammography and ultrasound in each group for BI-RADS 3, 4, and 5 was calculated. PPV of individual morphological descriptors were also calculated. For sensitivity calculation of the BI-RADS score, BI-RADS 1, and 2 were not included. BI-RADS 3 lesions in which histopathology was not done were also not included. The association of various morphological descriptors with malignancy was analyzed using a chi-square chart. p-value of less than 0.05 was considered significant.
Out of 216 patients, 214 (99.0 %) patients were females and 2 (0.9 %) patients were male. Female to male ratio was 107:1. The age of the patients ranges from 25 to 83 years with a mean age of 52.2 + 11.2 SD years. The age distribution of patients is shown in Figure 1:
Out of total patients, 27 (12.5 %) had a positive family history of breast carcinoma. 139 (64.4 %) patients were post-menopausal and 75 (34.7 %) patients were premenopausal. 96 (44.4%) patients had screening mammography and 120 (55.6%) patients presented with breast-related symptoms. 76 (35.2%) patients presented with breast lumps, 64 (29.6%) patients had pain, and 13 (6.0%) patients had nipple discharge. 7 (3.2%) had nipple retraction and 9 (4 .1%) had skin changes. Regarding breast parenchymal density, 42 (19.4%) had fatty, 82 (38%) patients had fibroglandular, 79 (36.6%) patients had heterogeneously dense and 13 patients (3.0%) had dense breast parenchyma (Table 1).
|
Breast parenchymal density |
No. of patients (n=216) (percentage) |
No. of patients with malignant disease involvement (percentage) |
No. of patients with benign disease involvement (percentage) |
PPV Malignant/total lesions (percentage) |
|
Fatty |
42 (19.4%) |
15 (24.6%) |
1 (12.5%) |
15/16 (93.7%) |
|
Fibroglandular |
82 (38.0%) |
20 (32.7%) |
3 (37.5%) |
20/23 (86.9%) |
|
Heterogenously dense |
79 (36.6%) |
20 (32.7%) |
4 (50.0%) |
20/24 (83.3%) |
|
Dense |
13 (6.0%) |
6 (9.8%) |
0 (0.0%) |
6/6 (100%) |
|
Total |
216 (100%) |
61 (100%) |
8 (100%) |
50 patients had breast-related complaints in the right breast, 56 had in the left breast and 7 patients report bilateral breast symptoms. The involved quadrant in breast imaging is tabulated in Table 2. Distribution of patients according of BI-RADS category is shown in Table 3.
|
Quadrant of breast involved |
Total no. of patients |
No. of patients with malignant disease involvement (n=61) |
No. of patients with benign disease involvement (n=8) |
|
|
Upper outer |
47 |
31 |
5 |
|
|
Retroaerolar |
20 |
11 |
3 |
|
|
Upper inner |
13 |
8 |
0 |
|
|
Lower outer |
5 |
5 |
0 |
|
|
Lower inner |
5 |
4 |
0 |
|
|
All quadrants |
3 |
2 |
0 |
|
BI-RADS category |
Frequency |
Percentage |
Valid percentage |
Cumulative percentage |
|
1 |
14 |
6.5 |
6.5 |
6.5 |
|
2 |
106 |
49.1 |
49.1 |
55.6 |
|
3 |
29 |
13.4 |
13.4 |
69 |
|
4A |
6 |
2.8 |
2.8 |
71.8 |
|
4B |
3 |
1.4 |
1.4 |
73.1 |
|
4C |
36 |
16.7 |
16.7 |
89.8 |
|
5 |
22 |
10.2 |
10.2 |
100 |
|
Total |
216 |
100 |
100 |
In 69 patients with suspicious imaging findings, the biopsy was performed. 62 (89.9%) patients presented with breast lumps, 24 (34.8%) patients had pain, and 8 (11.6%) patients had nipple discharge. 6(8.7%) had nipple retraction and 7 (10.1%) had skin changes. 36 (52.2%) patients were post-menopausal and 32 (46.1%) patients were premenopausal. 1(1.4%) patient was male. On histopathology, 61 (88.4%) patients had malignant disease and 8 (11.5%) were having benign lesions. Distribution of breast lesions according to BI-RADS category are shown in Table 4. The mean age of patients with malignant masses was 50.9 years + 13.1 SD (Age range: 28-83 years). No significant statical difference is noted between younger and older groups (p-value 0.397). Out of 61 patients with malignant lesions, 8 (11.6%) had a positive family history of breast carcinoma. The mean size of the malignant mass was 3.0 cm + 1.8 SD (Size range: 0.3-10.0 cm). The sensitivity of combined mammogram and ultrasound was calculated to be 98.3%, specificity was 25.0%, PPV was 90.9%, NPV was 66.6% and diagnostic accuracy was 89.9%.
|
Histopathology |
BI-RADS category |
Total |
p-value |
||||
|
3 |
4A |
4B |
4C |
5 |
|||
|
Malignant |
1 |
2 |
2 |
34 |
22 |
61 (88.4%) |
|
|
Benign |
2 |
3 |
1 |
2 |
0 |
8 (11.6%) |
0 |
|
Total |
3 |
5 |
3 |
36 |
22 |
69 (100%) |
|
The left breast was most commonly involved (57.3 %.) The upper outer quadrant was the commonest site of disease involvement observed in 50.8% of cases. The distribution and association of morphological features of malignant lesion on mammography and ultrasound with the BI-RADS category are tabulated as follows (Table 5).
|
Morphological features on imaging |
BI-RADS Category |
Positive predictive value |
p-value |
||||
|
3 |
4A (n=5) |
4B (n=3) |
4C (n=36) |
5 (n=22) |
(Malignant/Total) |
||
|
(n=3) |
|
||||||
|
Mammography |
|||||||
|
Mass margins |
|||||||
|
Spiculated |
0 (0.0%) |
0 (0.0%) |
1 (33.3%) |
18 (50.0%) |
8 (36.3%) |
27/27 (100%) |
N/A** |
|
Indistinct |
0 (0.0%) |
1 (20.0%) |
0 (0.0%) |
6 (16.6%) |
3 (13.6%) |
8/9 (88.8%) |
0.09 |
|
Masked |
0 (0.0%) |
0 (0.0%) |
1 (33.3%) |
3 |
1 (4.5%) |
5/5 (100%) |
N/A** |
|
-8.30% |
|||||||
|
Microlobulated |
0 (0.0%) |
2 (40.0%) |
0 (0.0%) |
4 (11.1%) |
7 (31.8%) |
12/13 (92.3%) |
0.05 |
|
Circumscribed |
2 (66.6%) |
2 |
1 (33.3%) |
5 (13.8%) |
3 (13.6%) |
8/13 (92.3%) |
0.111 |
|
-40.00% |
|||||||
|
Mass Shape |
|||||||
|
Irregular |
0 (0.0%) |
2 (28.5%) |
2 (66.6%) |
26 (72.2%) |
17 (77.2%) |
44/47 (93.6%) |
0 |
|
Oval |
1 (33.3%) |
1 (20.0%) |
0 (0.0%) |
5 (13.8%) |
2 (9.0%) |
7/9 (77.7%) |
0.224 |
|
Round |
1 (33.3%) |
2 (40.0%) |
1 (33.3%) |
3 (8.3%) |
3 (13.6%) |
7/10 (70.0%) |
0.107 |
|
Microcalcifications |
0 (0.0%) |
0 (0.0%) |
2 (66.6%) |
19 (52.7%) |
10 (45.4%) |
31/31 (100%) |
0.007 |
|
Skin thickening |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
9 (25.0%) |
14 (63.6%) |
23/23 (100%) |
0.03 |
|
Architectural distortion |
0 (0.0%) |
1 (20.0%) |
2 (66.6%) |
28 (77.7%) |
21 (95.4%) |
49/52 (94.2%) |
0.008 |
|
Intramammary lymphnodes |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
1 (2.7%) |
0 (0.0%) |
1/1 (100%) |
0.814 |
|
Ultrasound |
|||||||
|
Echogenicity |
|||||||
|
Hypoechoic |
1 (33.3%) |
4 (80.0%) |
3 (100%) |
28 (77.7%) |
20 (90.9%) |
50/56 (89.2%) |
0.001 |
|
Isoechoic |
1 (33.%) |
1 (20.0%) |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
0/2 (0.0%) |
N/A* |
|
Heterogenous |
1 |
0 (0.0%) |
0 (0.0%) |
8 (22.2%) |
2 (9.0%) |
11/11 (100%) |
N/A** |
|
-33.30% |
|||||||
|
Margins |
|||||||
|
Well-defined |
2 (66.6%) |
3(60.0%) |
0 (0.0%) |
2 (5.5%) |
2 (9.0%) |
5/9 (55.5%) |
0.098 |
|
Irregular |
1 (33.3%) |
2 (40.0%) |
2 (66.6%) |
19 |
15 (68.1%) |
37/39 (94.8%) |
0.001 |
|
-52.70% |
|||||||
|
Microlobulated |
0 (0.0%) |
0 (0.0%) |
1 (33.3%) |
11 (30.5%) |
2 (9.0%) |
12/14 (85.7%) |
0.727 |
|
Spiculated |
0 (0.0%) |
0 (0.0%) |
0 (0.0%) |
4 (11.1%) |
3 (13.6%) |
7/7 (100%) |
N/A** |
|
Vascularity |
2(66.6%) |
3 (60.0%) |
1 (33.3%) |
29 (80.5%) |
19 (86.3%) |
48/53 (90.5%) |
0 |
|
Axillary lymphadenopathy |
1 (33.3%) |
1 |
2 (66.6%) |
29 (80.5%) |
18 (81.8%) |
38/51 (74.5%) |
<0.0001 |
|
-20.00% |
|||||||
|
N/A* All benign, N/A** All malignant. |
|||||||
Few cases of patients with breast lesion with either benign and malignant lesion in whom histopathology was performed are shown in Figure 2-6.
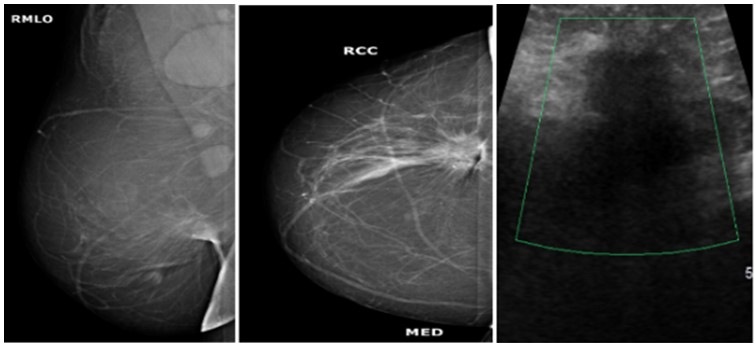 Figure 2: Mammogram MLO (a) and CC; (b) Views of a 57 years old woman showing spiculated mass in the lower outer quadrant of right breast with architectural distortion. Enlarged axillary lymphadenopathy was also noted. On ultrasound doppler images; (c) It appears as hypoechoic lesion with irregular margins, it was labelled as BI-RADS category 5 lesion. On histopathology, it turns out to be malignant infiltrating ductal carcinoma.
Figure 2: Mammogram MLO (a) and CC; (b) Views of a 57 years old woman showing spiculated mass in the lower outer quadrant of right breast with architectural distortion. Enlarged axillary lymphadenopathy was also noted. On ultrasound doppler images; (c) It appears as hypoechoic lesion with irregular margins, it was labelled as BI-RADS category 5 lesion. On histopathology, it turns out to be malignant infiltrating ductal carcinoma. 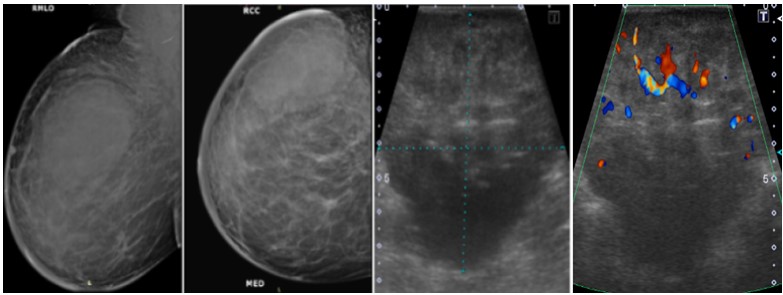 Figure 3: Mammogram MLO (a) and CC; (b) Views of a 29 years old woman showing large oval shaped mass with lobulated margins in the upper outer quadrant of right breast with overlying skin thickening. On ultrasound gray scale; (c) and doppler images ;(d), It appears as heterogenous lesion with irregular margins and increased vascularity, it was labelled as BI-RADS category 4C lesion and was diagnosed as invasive ductal carcinoma on histopathology.
Figure 3: Mammogram MLO (a) and CC; (b) Views of a 29 years old woman showing large oval shaped mass with lobulated margins in the upper outer quadrant of right breast with overlying skin thickening. On ultrasound gray scale; (c) and doppler images ;(d), It appears as heterogenous lesion with irregular margins and increased vascularity, it was labelled as BI-RADS category 4C lesion and was diagnosed as invasive ductal carcinoma on histopathology. 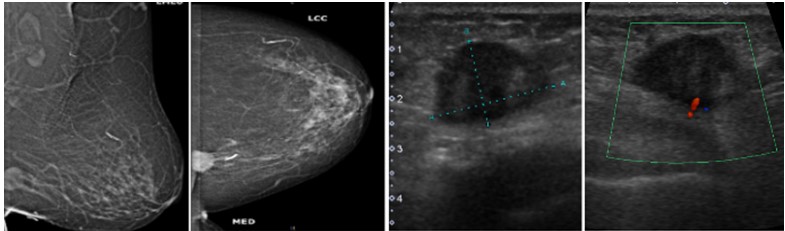 Figure 4: Mammogram MLO (a) and CC; (b) views of a 29 years old woman showing small rounded mass with well-defined margins in the lower inner quadrant of left breast. On ultrasound gray scale (c) and doppler images; (d) it appears as heterogenous lesion with microlobulated margins and minimal internal vascularity, it was labelled as BI-RADS category 4B lesion and was diagnosed as infiltrating ductal carcinoma on histopathology.
Figure 4: Mammogram MLO (a) and CC; (b) views of a 29 years old woman showing small rounded mass with well-defined margins in the lower inner quadrant of left breast. On ultrasound gray scale (c) and doppler images; (d) it appears as heterogenous lesion with microlobulated margins and minimal internal vascularity, it was labelled as BI-RADS category 4B lesion and was diagnosed as infiltrating ductal carcinoma on histopathology. 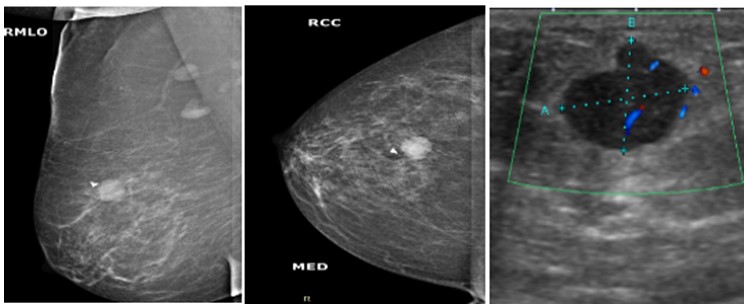 Figure 5: Mammogram MLO (a) and CC; (b) views of a 56 years old woman showing small ovoid mass with microlobulated margins in the upper outer quadrant of right breast. On ultrasound doppler images; (c) A well-defined hypoechoic lesion and internal vascularity is identified , it was labelled as BI-RADS category 4A lesion, On histopathology, it was diagnosed as micropapillary carcinoma.
Figure 5: Mammogram MLO (a) and CC; (b) views of a 56 years old woman showing small ovoid mass with microlobulated margins in the upper outer quadrant of right breast. On ultrasound doppler images; (c) A well-defined hypoechoic lesion and internal vascularity is identified , it was labelled as BI-RADS category 4A lesion, On histopathology, it was diagnosed as micropapillary carcinoma. 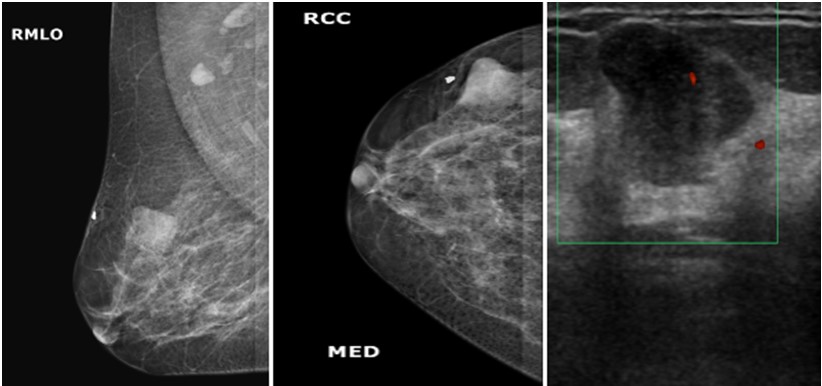 Figure 6: Mammogram MLO (a) and CC; (b) views of a 30 years old woman showing well defined soft tissue density mass in the upper outer quadrant of right breast with focal indistinct medial margins. On ultrasound doppler images (c); it appears as well-defined hypoechoic lesion with microlobulated margins and minimal internal vascularity, it was labelled as BI-RADs category 4A lesion. On histopathology, it turns out to be benign phyllodes tumor.
Figure 6: Mammogram MLO (a) and CC; (b) views of a 30 years old woman showing well defined soft tissue density mass in the upper outer quadrant of right breast with focal indistinct medial margins. On ultrasound doppler images (c); it appears as well-defined hypoechoic lesion with microlobulated margins and minimal internal vascularity, it was labelled as BI-RADs category 4A lesion. On histopathology, it turns out to be benign phyllodes tumor. Breast cancer is one of the most common cancers worldwide. The early detection and screening of breast cancer enable to reduce cancer-specific mortality. Ghaemian N et al. studied the sensitivity and specificity of mammography and ultrasonography alone as well as combined mammography with ultrasonography in detecting malignant breast masses. They concluded that the combined mammography and ultrasonography have significantly higher sensitivity (84.9%) as compared to mammography (72.6%) and ultrasonography alone (68.9%) with an increase in overall sensitivity by 12.3%. [3]. However, the specificity for combined mammography and ultrasonography has decreased (43%) when compared to mammography (43.9%) and ultrasound (48.6%) alone [3]. Another study by Lee et al. report similar results when studying the performance of screening imaging devices in a cohort study [10]. A study by Berg WA et al. also showed similar results [11]. The results of our study showed the high sensitivity (98.3%) and low specificity (25.0%) of combined mammogram and ultrasound in detecting malignant breast masses. We believe that the low specificity in our study is attributed to the limited number of patients in the BI-RADS 3 category who underwent biopsy based on the physician’s decision or the patient’s discretion.
In mammography, breast density poses a significant risk factor in the misdiagnosis of breast cancer in young women who are less than 45 years of age, premenopausal females, or women with small breasts [12]. According to the literature, women with dense breast parenchymal density are 5 times more susceptible to develop breast cancer as compared to women with fatty involutional changes [13]. Performing concomitant ultrasound in addition to mammography aims to improve the effectiveness of imaging in the diagnosis of breast cancer especially in women with heterogeneously dense and dense breast parenchyma. [14]. Berg WA et al. emphasizes the role of ultrasound as an adjunct to mammography to improve the detection of breast cancer. He states that out of 37085 examinations, 127 (0.34%) cancers were detected on ultrasonography [15]. In our study, 1 patient has a negative mammogram with a complex heterogeneous area of ductal dilatation in the retroaerolar region and was assigned the BI-RADS category3 on imaging. On histopathology, it turns out to be a malignant lesion. This emphasizes the fact that the concomitant use of ultrasound with mammography improves cancer detection, especially in lesions that are not palpable or in patients with dense breasts where breast density may mask the small lesions.
Estimation of the tumor size is important in regards to deciding the treatment strategy towards breast masses particularly about the use of adjuvant chemotherapy and breast conservation approach. In a study by Luparia et al., the median pathological tumor size was 2.2 cm [16]. Another study by Wasif N, et al. showed a mean tumor size of 2.1cm on mammography and 1.7 cm on ultrasonography [17]. Mohapatra SK et al. reported the median size of breast lesions to be 4.15 cm (range 1.2-9.5 cm) [2]. In our study, the mean size of breast lesions was 3.0 cm + 1.8 SD (range 0.3-10.0 cm). The larger tumor size at the time of detection may be attributed to the low breast cancer awareness and lack of education regarding the importance of self-examination and screening programs in the population.
Positive family history of breast cancer is considered an important risk factor for the subsequent development of breast cancer particularly in women having breast cancer in first-degree relatives. Familial breast cancers usually occur due to genetic mutations at the molecular level in breast cancer type 1 and 2 genes (BRCA1 and BRCA2 respectively) [18]. Seiffert K, et al. concluded in their study that there was a higher detection rate of breast cancer (48.6%) in patients with first-degree relatives and 42.7% in patients with second-degree relatives [19]. In our study, 11.6 % of patients had a family history of breast cancer.
Our study found a significantly higher frequency of breast cancer in both pre and post-menopausal women (46.1 % and 52.2% respectively) without a significant statical difference (p-value 0.22). This is contrary to the results of previous studies done by Mohapatra SK, et al. [2] and Surakasula, et al. [20] which establish a significant relationship between postmenopausal status with malignancy (p-value 0.001) The results of our study are in concordance with the findings of study by Heer E et al. which showed proportionally greater age standardized incidence as well as mortality rates of breast cancer in both premenopausal (40.6%) and post-menopausal (59.4%) group in the countries with low and middle United Nations Development Programme (UNDP) Human Development Index (HDI) as compared to countries with high HDI [21].
Regarding the morphological features of breast lesion on mammography and ultrasonography, various features such as spiculated (100% of malignant tumors), and microlobulated margins (p-value 0.05), irregular shape (p-value 0.000), presence of microcalcifications (p-value 0.007), skin thickening (p-value 0.03), architectural distortion (p-value 0.008), and vascularity (p-value 0.000) have significant correlation with malignancy. On ultrasonography, most malignant lesion tends to be hypoechoic (p-value 0.001) or heterogeneous (100% of malignant tumors). All isoechoic lesions were benign on histopathology. Out of 61 malignant lesion, 8 (13.1 %) malignant lesions show benign features such as circumscribed margins (p-value 0.111) on mammography and 5 (8.1%) show well defined margins on ultrasonography (p-value 0.001), these results were similar to that of earlier studies [2,22]. This finding needs consideration when evaluating breast lesions to minimize the chances of misdiagnosis of malignant breast lesions as benign.
The limitations of this study are that the non-randomized consecutive sampling was done with small sample size which may alter the accuracy of our results. Moreover, patients with benign lesion in BI-RADS category 3 in which histopathology was not performed were excluded, which may cause bias in the calculation of results.
We conclude that the combined mammography and ultrasound serve as an important diagnostic tool, both for screening purpose as well as in patients with breast related symptoms for the diagnosis of breast cancer. Moreover, the morphological descriptors of malignancy on mammography and ultrasound as described by BI-RADS lexicon are reliable indicators of malignancy in patients with breast lesions.
None.
