 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Aliya Ishaq1* , Muhammad Jamshaid Husain Khan2, Farah Ibrahim Bakhit Juma1, Lizica Itu1, Sameera Naureen3, Nisha Kunal1, Yasir aminabdellatif3, Arfan Al Awa4, Zaid Abdulaziz4
, Muhammad Jamshaid Husain Khan2, Farah Ibrahim Bakhit Juma1, Lizica Itu1, Sameera Naureen3, Nisha Kunal1, Yasir aminabdellatif3, Arfan Al Awa4, Zaid Abdulaziz4
1Specialist General Surgeon, Dubai Hospital, Dubai, UAE
2Specialist, Internal Medicine, Dubai Hospital, UAE
3Senior Specialist General Surgeon, Dubai Hospital, UAE
4Consultant General and Breast Surgeon, Dubai Hospital, UAE
Correspondence to: Aliya Ishaq, Specialist General Surgeon, Dubai Hospital, Dubai, UAE
Received date: August 04, 2022; Accepted date: Spetember 01, 2022; Published date: September 07, 2022
Citation: Ishaq A, Khan MJH, Juma FIB, et al. Cecal Perforation: Gastrointestinal Menifestation of Acute Lymphoblastic Leukemia. J Med Res Surg. 2022; 3(S2): 12-16. 10.52916/jmrs22S204
Copyright: ©2022 Ishaq A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Background: Hematological malignancies present with gastrointestinal manifestations in the form of typhlitis, colitis and bowel perforation. Prompt diagnosis and appropriate treatment of these entities is essential because they are associated with high morbidity and mortality. Case report: We present a case report of a young female patient who was diagnosed with acute lymphoblastic leukemia and while being on induction chemotherapy started having fever, pneumonia, positive blood culture and was started for that on broad spectrum antibiotics after which she developed abdominal pain and loose motion and was found to have clostridial difficile a toxin positive in blood. Surgical consult was taken for non-settling abdominal pain. It was a challenging diagnosis as patient was having loose motion with positive clostridial difficile further more ct scan abdomen done with contrast showed only bowel thickening which was in favor of colitis along with ascites. She was initially managed conservatively and ascitic diagnostic tap also was done which showed serous fluid. However, her persistent abdominal pain which was not settling led her to go another ct scan abdomen after 3 days of initial ct scan and showed specks of free air around cecum based on which she was taken to operation theatre and was found to have big cecal perforation with fecal peritonitis, she ended up having right hemicolectomy and ileo transverse stoma formation. She had prolonged Intensive Care Unit (ICU) stay but eventually recovered fully and was shifted to general ward where after wound healing was taken over by hematology department for continuation of her chemotherapy. Final histopathology of right hemicolectomy specimen showed focal marked mucosal ulcerations/erosions with patchy submucosal neutrophilic abscesses with fibrinosuppurative necrosis, and marked serositis with dense acute (fibrinopurulent) inflammation, all bowel layers mucosa, sub mucosa, muscularis and serosa showed neutrophilic infiltrates, there was no evidence of pseudomembranous colitis, granuloma or malignancy. Conclusion: Patients on chemotherapy for hematological malignancies are neutropenic and are at high risk of bowel ischemia and perforation emanating to there primary disease, immunocompromised status and direct and indirect side effects of chemotherapeutic agents. A high index of suspicion is needed to diagnose these cases accurately and treat accordingly to prevent mortality.
Gastrointestinal manifestation of hematological malignancies, Neutropenic enterocolitis, Typhlitis, Leukemia, Chemotherapy
Gastrointestinal manifestations are seen in 20-24% [1] patients diagnosed with leukemia. Early diagnosis and treatment is essential for patient’s survival as these patients are immunocompromised and can deteriorate rapidly leading to fatal out come [2]. Laboratory and imaging modalities should be used as adjuncts to help in early diagnosis [3]. Indications for surgery are same as for non leukemic patients mailly obstruction, perforation and bleeding but the out come is worse as compare to non leukemic patients and morbidity and mortality rates are high [4].
28 years old previously healthy female was admitted in peripheral hospital with syncope, seizures and shock, she was having previous history of generalized weakness for many days. Her blood investigations done in peripheral hospital showed severe Thrombocytopenia (20,000) and Anemia ( Hb of 1.3 g/ dl ) with Elevated White Blood Cells (WBCs) (120,000 10^3/uL).
Patient was admitted to ICU and received Multiple Blood transfusions with Fresh Frozen Plasma (FFP). Labs also showed significantly deranged coagulation profile, high D-dimer but normal fibrinogen. Presence of fragmented Red Blood Cells (RBCs) also present.
Blood film showed lymphocytes markedly increased with condensed partially open chromatin, scant cytoplasm and single nucleoli. Fair number of smudge cells seen, few number of plasma cells seen. Overall findings were suggestive of chronic lymphoproliferative disorder and therefore she was referred to our hospital being a tertiary care facility for work up of lymphoproliferative disorder.
She was diagnosed in our hospital by hematology team with acute lymphoblastic leukemia with Central Nervous System (CNS) involvement severe thrombocytopenia (20,000) and anemia (Hb of 1.3 g/dl ) with elevated WBCs (120,000 10^3/uL).
Her bone marrow trephine biopsy showed markedly hypercellular marrow which is heavily infiltrated with small to medium sized blasts, findings are consistent with acute T cell lymphoblastic Leukemia/lymphoma which has been confirmed by immunophenotyping study.
She was started on chemotherapy (HyperCVAD) and during induction therapy on 19th post admission, she developed febrile neutropenia secondary to bacteremia K. pneumoniae NDM (Colistin R broth-dilution 16, Tigecycline S higher MIC 2, Meropenem MIC 16), and co-infection with C. difficcile that lead to membranous colitis.
On 28th post admission day she started having abdominal pain and loose motion and clostridium difficile blood toxin came positive and she was treated for that however, pain and loose motion did not settle so surgical consult was obtained.
When examined by surgical team she was septic with abdominal distension with clinical ascites as well as generalized tenderness so Computed Tomography (CT) scan abdomen with contrast was requested with ongoing resuscitation and ICU involvement to rule out hollow viscus perforation.
Hepatosplenomegaly, mild to moderate ascites. Mucosal thickening and distended cecum and ascending colon no oral contrast, most likely suggesting colitis. Lower cuts through lung were also suggestive of pneumonia as well as pericardial effusion. Keeping in mind positive clostridial difficile toxin, pan cytopenia, loose motion and CT scan findings patient was not taken for exploration but since patient was having peritonitis with sever sepsis we decided to go for diagnostic ascites with a view that if diagnostic tap will show bowel contents will proceed for exploration (Figure 1-5).
Ascitic fluid was tapped and it was serous, which was sent for microbiology while management with broad spectrum antibiotics (Meropenum, based on blood culture) and clostridial difficile colitis was treated. Since ascitic tap did not show any evidence of bowel contents and clostridial difficile was positive in blood with ongoing loose motion, the clinical picture was attributed to pseudomembranous colitis and management was continued in that line with vancomycin, nill by mouth, nasogastric tube in place with gravity drainage and total parenteral nutrition with close observation but when patient did not improve and kept on having abdominal pain and distension with systemic sepsis and raised inflammatory markers it was decided to go for CT scan abdomen with oral and iv contrast on 31st post admission day to rule out any intrabdominal complication related to pseudomembranous colitis which showed increasing in the amount of large low density free intraperitoneal ascitic fluid.
Diffuse bowel wall thickening with submucosal edema involving the entire small and large bowel loops extending till the level of the splenic flexure. Small doubtful extra luminal air foci were seen anterior to the non opacified cecum raising possibility of sealed perforation (Figure 6-9).
Based on clinical findings and CT scan findings patient was taken to operation theatre and diagnostic laparoscopy was done which showed massive ascites turbid fluid with inflamed bowel and adhesions and fibrin so converted to laparotomy. On laparotomy aabdomen was full of ascites 7-8 liters with massive fibrinous deposits every where in the entire peritoneal cavity, inter bowel, pelvic, subhepatic, para splenic, para hepatic.
Small bowel was inflamed with congested mesentery.
Inflamed right colon with eaten up cecum with a big perforation loosely covered by omentum at its anterior wall with free fecal discharge leading to fecal peritonitis, gross appearance of cecal and colonic wall was suggestive of pseudomembranous colitis (Figure 10).
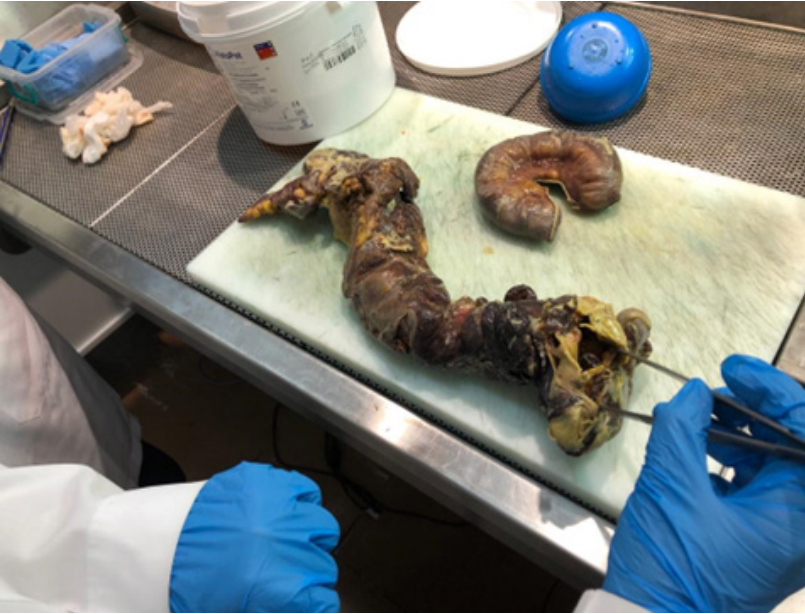 Figure 10: Right hemicolectomy specimen as seen in histopathology lab after
it was put in formalin,forceps showing area of cecal perforation, as evident
looks grossly as pseudomembranous colitis.
Figure 10: Right hemicolectomy specimen as seen in histopathology lab after
it was put in formalin,forceps showing area of cecal perforation, as evident
looks grossly as pseudomembranous colitis.
Right hemicolectomy was done and ileum and transverse colon brought out together while posterior wall was stitches and anterior kept open as stoma. Two large bore pelvic and right paracoloic drains were placed and abdomen closed.
Patient was shifted to intensive care unit, mechanically ventilated and was started on inotropic support.
Later on peritoneal fluid culture showed carbapenems producer Klebsiella pneumonia. Patient was started on antibiotics as per culture.
Patient stayed in ICU for 2 weeks was gradually weaned off and was started on ng feed, stoma started functioning and she later developed surgical side wound infection and wound was opened up and was put on vacuum dressing.
After 2 weeks she was shifted to high dependency and then general ward, she improved hemodynamically as well as biochemically and also developed some abdominal collection which was drained under radiological guidance, fluid culture grew vancomycin resistant Enterococcus faecium and antibiotics started as per culture.
Histopathology of appendix, small bowel,cecum, ascending and transverse colon showed-focal marked mucosal ulcerations/ erosions with patchy submucosal neutrophilic abscesses with fibrinosuppurative necrosis, and marked serositis with dense acute (fibrinopurulent) inflammation, all bowel layers mucosa, sub mucosa, muscularis and serosa showed neutrophilic infiltrates, there was no evidence of pseudomembranous colitis, granuloma or malignancy.
She required intensive in put from physiotherapist and stoma care nurses to make her up and about and to let her learn stoma care. Her wound was closed after 2 months and patient was taken over by hematology team for further management of her acute lymphoblastic leukemia. She is currently in hematology ward undergoing chemotherapy for acute lymphoblastic leukemia and has not yet achieved remission.
Gastrointestinal complication related to leukemia can be etiologically divided in to three categories [5].
Most common of these is leukemic infiltrates, they present as leukemic infiltrate can produce plaquelike thickening of the bowel wall, raised nodular lesions, diffuse mucosal and subrnucosal infiltration, polyp formation, and ulceration [6].
Chemotherapy may directly produce necrosis and weakened areas in the bowel wall while destroying the underlying malignant cells. Bacterial, fungal, or viral overgrowth then can invade the bowel and produce ulceration, pseudo membrane formation, or perforation. Steroids, antimetabolites, and folic acid antagonists allow development of a granulocytic lesions, especially in the cecum [7].
Some chemotherapeutic agents produce a direct neurotoxic effect on the gut. Vincristine in particular may incite a reversible megacolon, which probably results from autonomic ganglia damage [8].
In our case diagnosis was delayed because the presentation was more like pseudomembranous colitis specially when we were having positive clostridial difficile toxin in blood with loose motion. Further more CT scan showed diffuse inflammation of small and large bowel with ascites with out any evidence of perforation and even being clinically suspicious when ascitic tap was done it turned out to be as serous with out any fecal contents, it was only strong suspicion and deteriorating patients condition that repeat CT scan after 5-6 days of abdominal pain showed some isolated pockets of extraluminal air at cecal wall. However it can not be said with certainty weather patient was hiving perforation at time of first CT scan or not or may be persistent inflammation, ischemia and immunocompromised status of patient lead to perforation later on.
Further more the final histopathology for our patient showed non specific inflammation, it did not show any evidence of malignant infiltrates, neither granuloma, nor evidence of neutropenic colitis or pseudomembranous colitis as histopathology showed involvement of all layers of bowel with neutrophilic infiltrates with fibrinosuppurative necrosis which is not fitting in any of etiological factors responsible for colitis in leukemic patients.
Our patient was chemotherapeutic agents which were started 3 weeks before she developed abdominal pain and at time of abdominal pain her wbc count was 0.5 with absolute neutrophilic count of 0.9. Before developing abdominal pain she developed febrile neutropenia secondary to bacteremia K. pneumoniae for which she was started on broad spectrum antibiotics after which she developed diarrhea and got positive clostridial difficile toxin. It might explain that in our patient because of immunocompromised status of patient and low hemoglobin along with direct and indirect side effects of chemotherapeutic agents as she was on vincristine and doxorubicin both of which are pron to cause gastrointestinal side effects specially typhlitis and ischemic necrosis of cecum [8]. The cecum is particularly susceptible to injury because of its relative stasis, easy distensibility, predisposition toward mucosal ischemia, and normal high bacterial count [9].
These bacteria can grow in absence of neutrophils and can lead to ischemia and perforation, more ever these patients are prone to stasis because of immobility associated with severity of disease. In our case there was delay in intervention because of failure to detect hollow viscus perforation on CT scan and negative ascitic tap but fortunately recovered completely despite the fact that she was severely immunocompromised with fecal peritonitis and systemic sepsis. Her recovery took two months but it was expected.
Clinical scenario of our patient mimics neutropenic enterocolitis with is characterized by abdominal pain, fever, neutropenia and CT scan evidence of bowel wall thickening in an immunocompromised patient on chemotherapy for hematological malignancies but histopathological features which is gold standard of diagnosis excludes it as our patients histopathology showed involvement of all mucosal walls with neutrophilic infiltrates and characteristic histopathological feature of neutropenic enterocolitis is absence of neutrophilic infiltrates.
Patients with hematological malignancies are more pron to get gastrointestinal menifestations in the form of abdominal pain, fever, loose motion, obstruction or perforation. A high clinical suspicion is needed and appropriate laboratory and radiological studies should be done to rule out seroous complications and need for intervention to prevent high morbidity and mortality associated with delay.
This case report was written and images of patient’s scans were added after taking consent from patient and patient’s identity is not shown here and will be kept confidential.
No.
The autors declare that they have no conflict of interests.
