 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Roya Mehdikhani1* , Gholam Reza Olyaei2, Mohammad Reza Hadian2, Saeed Talebian Moghaddam2, Azadeh Shadmehr2
, Gholam Reza Olyaei2, Mohammad Reza Hadian2, Saeed Talebian Moghaddam2, Azadeh Shadmehr2
1Assistant Professor of Physical Therapy, Zanjan University of Medical Sciences (ZUMS), School of Allied Medical Sciences, Iran
2Physical Therapy Department, School of Rehabilitation, Tehran University of Medical Sciences and Health Services, Iran
Correspondence to: Roya Mehdikhani, Assistant Professor of Physical Therapy, Zanjan University of Medical Sciences (ZUMS), School of Allied Medical Sciences, Iran
Received date: August 04, 2022; Accepted date: August 23, 2022; Published date: August 29, 2022
Citation: Mehdikhani R, Olyaei GR, Hadian MR, et al. Assessment of Upper Trapezius Muscle Fatigue in Subclinical Myofascial Pain Syndrome Participants Versus Healthy
Control by JASA Method. J Med Res Surg. 2022; 3(4): 79-85. doi: 10.52916/jmrs224085
Copyright: ©2022 Mehdikhani R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Background: Myofascial trigger points are most commonly occurs in the upper trapezius, which is the
highest sensitive muscle in the body. Joint Analysis of Electromyography (EMG) Spectrum and Amplitude (JASA) method was applied to evaluate the occurrence of muscular fatigue during consecutive gripping exertions.
Methods: 64 right handed subjects took part in this study. Prior to the fatigue test, the maximal voluntary
contraction was measured three times. A force gauge was used for force measurement while recording with a monitor. Measurement was initiated with a Maximum Voluntary Contraction (MVC) force of the trapezius.
Results: After accomplishing fatigue protocol, they showed signs of exhaustion; however, they were not
subjectively evaluated for fatigue. As the protocol aimed at assessing muscle fatigue, a force level of 80% MVC was induced.
Conclusion: As revealed by the plots, the lower left quadrant could be defined as having an ‘‘force
reduction’’ rather than a ‘‘fatigue’’ trait based on the natural pushing up characteristics based at the
JASA plot definition, muscle fatigue or force reduction become manifested by way of above 90% of the
20 dots after fatigue test. Through the JASA method, researchers could gain insight in to the muscular
fatigue condition as well as the possible underlying mechanisms.
Upper trapezius muscle, Myofascial trigger point, Electromyography, Fatigue, Maximum voluntary contraction
JASA: Joint Analysis of Spectrum and Amplitude; MPS: Myofascial Pain Syndrome; MTrPs: Myofascial Trigger Points; PPT: Pressure Pain Threshold; TrPs: Trigger points; ATP: Adenosine Triphosphate; EMG: Electromyographic; SCNP: Subclinical Neck Pain; RMS: Root Mean Square; MPF: Mean Power Frequency; BMI: Body Mass Index; MVC: Maximal Voluntary Contraction; ANOVA: Analysis of Variance; MU: Motor Units; CV: Conduction Velocity.
Pain in the shoulder and cervical region, is one of the prevalent musculoskeletal problems among working population [1]. Origin of the pain in the Myofascial Pain Syndrome (MPS) is from Myofascial Trigger Points (MTrPs) which are the sensitive areas in taut bands of skeletal muscle [2]. According to Simons (1996), MTrPs develop due to stretching, contraction, or compression within a taut band resulting in a distinct pattern of pain.
Majority of MTrPs are usually found in the upper trapezius which is considered to be the most sensitive muscle in the body with the lowest Pressure Pain Threshold (PPT) [3].
This can be attributed to the constant work of the upper trapezius muscle to keep the head and neck in an erect position against the pull of gravity [4,5].
Individuals diagnosed with chronic upper trapezius myalgia have lower levels of adenosine triphosphate, adenosine diphosphate and phosphocreatine. However, they possess larger type-I fibers [6].
These changes lead to short-term hypoxia that brings about a limited energy crisis, often related to Trigger Points (TrPs) [7]. MTrPs can be either active or latent. Patients exhibit local and referred pain symptoms due to active trigger points [8].
These alterations result in temporary hypoxia which causes limited energy crisis, often associated with TrPs [5-7]. Local and referred pain symptoms are identified by the patients caused by active trigger points. Latent TrPs and MTrPs are similar in the clinical features. However, the difference lies in the fact that the former is not responsible for pain symptoms or produces unfamiliar symptoms for the patient. Patients are also concerned about latent MTrPs since their presence causes a noticeable restriction in the range of motion, muscle fatigue and muscle weakness [9-11].
It is believed that several factors including muscle overuse, mechanical overload, or psychological stress activate MTrPs [11]. It is likely that factors like injury, overusing or overloading of muscle fibers shorten these muscles involuntarily. The local tissues lack oxygen and nutrient supply, so their metabolic demand is increases [12]. The integrated hypothesis theory believes that the abnormal depolarization of muscle motor endplates and sustained muscular contraction lead to localized “ATP energy crisis” [13]. It has been reported that MTrPs and joint impairments are clinically associated [6,7,14]. It is possible that the tension of taut bands in muscle results in joint hypomobility. Furthermore, joint dysfunction might activate TrPs through abnormal input [15]. It is believed that there is a link between segmental hypomobility at the C3-C4 zygapophyseal joint with MTrPs in the upper trapezius in patients suffering from neck pain [16].
The ability to assess the joint position is known as kinesthetic sensibility, which is vital to move the head, trunk, and extremities in coordination [17-21]. Intensified movement irregularities [15] and movement errors [21] while performing repositioning activities are the indicative of kinesthetic sensibility dysfunction which is observed in middle-aged individuals suffering from chronic neck pain. Nonetheless, variations in muscle spindle discharge [23] or in the central output of the nervous system [24] could be reduced by pain. So far, no research has been conducted on the Electromyographic (EMG) responses while performing the repositioning movements in the young neck pain patients. Studies have shown that muscle fatigue due to voluntary contractions stimulates compensatory strategies to maintain the motor output by adapting the initial multi-joint organization; however, fatigue due to stimulated contractions does not lead to a segmental re-organization [25-27]. The results of fatigue-induced voluntary contractions are compensatory postural strategies to counteract or restrict the disturbance to postural control [28]. The central contribution provides certain sensory information, ignores and/or compensates for other data and enhances the motor output of postural control through development of the motor strategies [29,30]. It is believed that upper limb proprioception, motor patterns, and kinematics change through neck musculature fatigue [31]. This is probably because of the high density of sensory receptors in the neck muscles having neural links to the vestibular and oculomotor systems and thus being involved in postural regulation [32,33]. Due to both fatigue and Subclinical Neck Pain (SCNP), researchers believe that the changed neck sensory input is associated with changed upper limb motor control [33,34]. If neuromuscular electrical stimulation induces fatigue, the selective mechanism of sensory information is not triggered and thus, there will be no feedforward postural control [17,35]. It was assumed that the head and neck kinematics are altered due to presence of trigger points in upper trapezius muscle among patient group and kinematics in the SCNP group is altered in a different way because of neck muscle fatigue [36].
In one previous study, the joint Analysis of EMG Spectrum and Amplitude method was applied for evaluating the occurrence of muscular fatigue during consecutive gripping exertions. The pair of normalized RMS and MPF slopes was displayed in 2D JASA plots, while their changes over time were plotted along the x- and y-axis, respectively. The following 4 classes illustrated the results of their JASA plots [37]:
The current research study is a randomized controlled clinical trial. The research sample consisted of students and patients with neck pain referring to the physical therapy clinic of the faculty of rehabilitation. Ethical clearance was obtained from the Tehran University of Medical Science Ethics Committee and was adhered to throughout the study. Ethics committee number IR.TUMS.VCR.REC 1395.803;2016/10/22 and all subjects signed an in informed consent from prior to their inclusion. IRCT2017011426346N2, Grant number is 95 04 32 33402.
Sixty-four right-handed subjects (31 males and 33 females) [mean (standard deviation) age, 27.04 (2.3) years, Body Mass Index (BMI) 21.33 (1.05), height 169.23 (11.65) cm, weight 64.01 (5.2) kg] without upper extremity disorders took part in this study. The independent sample t-test was applied to match the age, weight, height, and BMI.
The study was approved by the local Ethics Committee number IR.TUMS.VCR.REC 1395.803; 2016/10/22 and all subjects signed an informed consent form prior to their inclusion.
Subjects for this research were selected among the undergraduate students who were referred to the physical therapy clinic of Tehran University of Medical Sciences after diagnosed with neck pain.
Exclusion criteria [11,20] and inclusion criteria [39] are in the Table 1.
| Inclusion criteria | Exclusion criteria |
1. Diagnosis of MPS in the upper Major criteria:
Minor criteria:
2. Aged 18-60 y No treatment, including injection, dry needling, and physical modalities, in the past 3 months No pain relievers in the past 48 h |
|
Eight-channel EMG system (Data Log P3 × 8, Biometrics Ltd., Gwent, UK) (CMRR: 496 dB at 60 Hz, input impedance 41012Ω, gain:1000 and band-pass filter: 20 Hz low cut-off, 450 Hz high cut-off).
Electrodes:Integral dry reusable electrodes (SX230, Biometrics Ltd., Gwent, UK) (Diameter: 10 mm, bipolar configuration and inter-electrode distance: 20 mm).
The electrode was inserted 2 cm near the midpoint of the line between the C7 spinous process and the acromion process [36].
The inter electrode distance (center to center) was 20 mm. Prior to electrode placement; skin impedance was decreased by shaving and washing the skin with 70% alcohol pads. A sampling rate of 1000 Hz and a filtered band passed at 20-480 Hz (amplified with a common mode rejection ratio >110 dB, overall gain 1,000, noise <1 mV RMS were used.
As described by Cram, surface recording electrodes were placed over the muscles [37] while the ground electrode was placed on the ipsilateral wrist.
For accomplishing the evaluations, the volunteers were comfortably seated in a chair, while their feet, hips and knees, buttocks, and arms were positioned on a digital balance in a flat state, flexed against the back of the chair at 90°, and relaxed at the sides of their bodies, respectively. They were asked to look forward in the same positions with their heads as their vertebral columns and trunks, and make no cervical and trunk rotations, flexions, or extensions during the tests. They were tested with unclothed shoulders; Figure 2 shows the experimental setup.
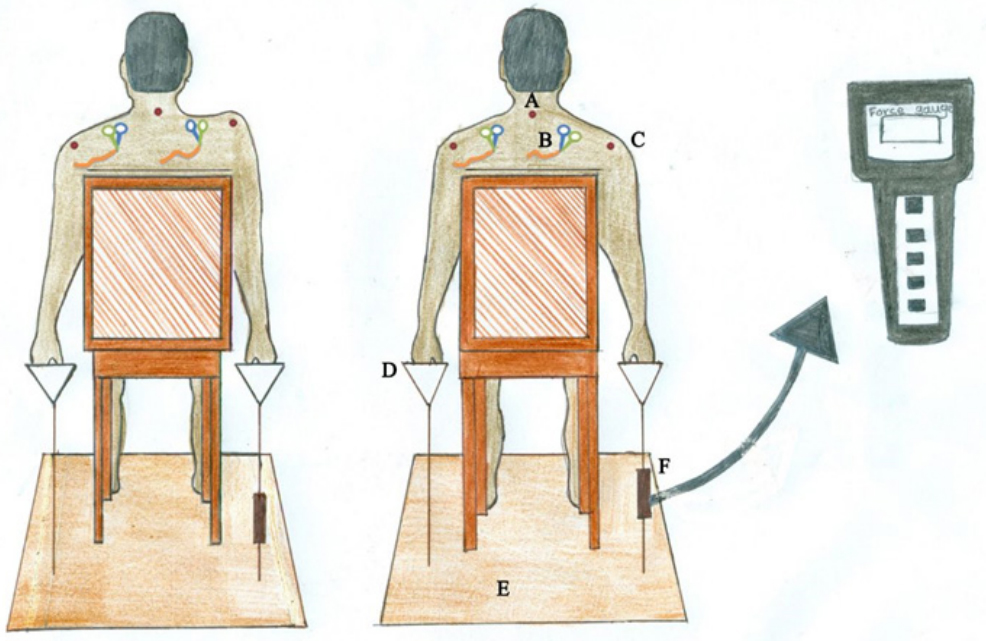 Figure 2: Details of experimental setup. Right image: A): First position B):
Surface electrode C): Acromion process D): Handle of set up E): Flat iron disc
F): Force gauge. Left image: Elevation of dominant shoulder against the load.
Figure 2: Details of experimental setup. Right image: A): First position B):
Surface electrode C): Acromion process D): Handle of set up E): Flat iron disc
F): Force gauge. Left image: Elevation of dominant shoulder against the load.
The diagnostic criteria described by Simons, et al. was used to determine the presence of latent MTrPs. An examiner with more than 10 years' experience performed the MTrPs diagnosis, and the criteria included the following:
Good inter-examiner reliability (k), which was ranging from 0.84 to 0.88, was found for the above mentioned criteria [12].
The Maximal Voluntary Contraction (MVC) was measured three times before doing the fatigue test. Each test lasted for about 10 seconds and an interval of 20 seconds was maintained between tests. Using the obtained highest value, calculation of the submaximal level was performed at 80% of the MVC. Two minutes after performing the last MVC test, the fatigue test was conducted. The procedure included isometric contractions of the upper trapezius muscle in the subject's dominant side. After adjusting the subjects’ heights as they were sitting comfortably with their arms passively hanging along their bodies, the force transducers were connected to both acromions of their shoulders. Then, they were asked to raise their shoulders against the force sensors while maintaining trapezius muscle contractions. A force gauge (5020 model, Taiwan) was used for force measurement while recording with a PC (sampling rate of 100 Hz).
The Measurement was started by applying the MVC of the trapezius. The subjects were able to lift the dominant shoulder against the force transducer, using force as much as possible for at least a 10 second interval. The experimental protocol was performed three-fold with 20 second breaks. The MVC was set as the maximum force value. After a 5 minutes break, a sustained submaximal contraction of the trapezius was done. The subjects kept a unilateral 80% MVC isometric shoulder elevation until the force gauge monitor showed 50% MVC in at least 3 minutes. The feedback of the force level was applied. The perceived exertion was determined at the beginning and following each minute of contraction. The force and EMG signals were logged throughout the MVC and sustained contractions. It was trying to motivate the subjects orally during the fatigue tests. After completing the protocol, signs of exhaustion were observed in the subjects; still, subjective evaluation was not performed for the fatigue. Since the purpose of the protocol was evaluating the muscle fatigue, a force level of 80% MVC was applied.
The SPSS 16.0 software was used for data analysis. By pre-planned contrasts to the first baseline, repeated measures Analysis Of Variance (ANOVA) was conducted for each variable. ANOVA was used to determine the joint position sense between pre-and post-fatigue conditions (SPSS v19, IBM Corporation, Armonk, New York, USA). Statistical significance was set at p ≤0.05.
Based on the JASA plot definition, muscle fatigue or force reduction was manifested by above 90% of the 20 dots after fatigue test, Table 2 shows Summary of RMS and MDF distribution in the third quadrant of the JASA plot for upper trapezius muscle. Regarding the JASA plots, Figure 3 and Figure 4 represent the regression line slopes of the RMS and MDF (median frequency) for all the 64 subjects under study. Fatigue and force reduction was shown by the majority of the (RMS, MDF) dots (0.88% and 0.92%) falling onto the quadrants as revealed by the JASA plots of left upper trapezius muscle and (0.91% and 0.94%). Table 3 shows a comparison between RMS and MDF parameters. while no dot was seen which was belonged to the adaptation and fatigue quadrants.
Slope RMS Lt |
Slope Med Lt |
Slope RMS Rt |
Slope Med Rt |
| -0.6223 | -0.9831 | -0.31 | -0.8573 |
| -0.9403 | -0.9898 | -0.9958 | -0.8091 |
| -0.8091 | -0.8786 | -0.8996 | -0.9159 |
| -0.8402 | -0.7749 | -0.8338 | -0.8852 |
| -0.8714 | -0.7895 | -0.8914 | -0.8988 |
| -0.6202 | -0.8858 | -0.7939 | -0.9667 |
| -0.8269 | -0.9853 | -0.9438 | -0.884 |
| -0.9135 | -0.9839 | -0.9342 | -0.9599 |
| -0.9702 | -0.9788 | -0.9647 | -0.9897 |
| -0.9045 | -0.9941 | -0.9648 | -0.9849 |
| -0.9729 | -0.9598 | -0.974 | -0.9397 |
| -0.9158 | -0.8959 | -0.9725 | -0.9843 |
| -0.9266 | -0.8058 | -0.964 | -0.9799 |
| -0.9486 | -0.9524 | -0.9552 | -0.9786 |
| -0.9483 | -0.9863 | -0.9799 | -0.9843 |
| -0.9734 | -0.9684 | -0.9859 | -0.9893 |
| -0.8516 | -0.9813 | -0.9831 | -0.983 |
| -0.9601 | -0.9063 | -0.9876 | -0.9919 |
| -0.9072 | -0.8991 | -0.9932 | -0.8991 |
| -0.8553 | -0.9542 | -0.9932 | -0.9255 |
Also, a similar phenomenon happened to the plots of left and right upper trapezius muscles.
JASA of EMG allowed us to consider simultaneous changes in the spectra and amplitudes [39,40]. Our experimental JASA prototype proved that it is able to designate the association between EMG spectra and amplitudes, i.e., between RMS and MDF on the one side of upper trapezius and the force and fatigue on the other side [40].
Nevertheless, the complexities of the underlying physiological processes and mechanisms would make it very difficult to elaborately define the different quadrants based on the mentioned alterations. As revealed by the plots, based on the natural pushing up characteristics, the lower left quadrant could be defined as having a ‘‘force reduction’’ rather than a ‘‘fatigue’’ trait [41].
Generally, a ‘‘size principle’’ is sequentially followed from low- (type-I) to the high-threshold (type-II) Motor Units (MUs) in the recruitment order [36,37]. Moreover, a lower movement is associated with higher velocity in the MU recruitment and derecruitment thresholds [41,42]. Therefore, during the fatigue test, it was believed that the MU rotation had occurred after type-I MU recruitments and continuous discharges. Following the type-II MU participations in the contractions, withdrawal of some of the recruited type-I MUs had occurred at the beginning of the contractions, which had led to their inactivation. Compared to the slow muscle fibers, fast fibers, which are innervated by type-II MUs, are involved in a higher maximum rate of depolarization and repolarization and shorter action potential duration [32,36].
A larger MDF value resulted from a higher Conduction Velocity (CV) occurred during shorter action potentials, which provide EMG spectra with high-frequency components. Rate-coding can provide the benefits of changing the number of recruited MUs and modulating muscle force [41]. DeLuca and Erim suggested the existence of a negative correlation between MU recruitment threshold and firing rate in this process. This was implicative of lower mean firing rates of type-II MUs, which showed a high recruitment threshold. Therefore, the RMS values of lower than those recorded at the initial stage of the typing activities might be obtained due to the lower myoelectric signal amplitudes occurring at the electrodes. The adaptation conditions of the lower left quadrant muscles were deduced from the observations of the decreasing RMS and MDF in the JASA plots [26,30-32].
The inevitable type-II MU withdrawal at this stage was due to the long-term muscular contractions during the activities. The occasional back-substitutions of some inactive type-I MUs were related to their responsibilities for successive contractions. Another mechanism called synchronization occurred at this stage [17,20,22], the more dominant of its effect on the EMG recordings could be especially evidenced when only a few MUs were involved at low contraction levels. The additional enhancement of the power spectrum components occurring in the low-frequency ranged between 20-40 Hz with subsequent fatigue was documented by Hagg [42]. This situation could respectively provide an explanation for the positive and negative slopes for the RMS and MDF values of some muscles in the JASA method and their consequent categorization into the ‘‘fatigue’’ quadrant muscles in our study [43, 44].
Decreased and increased EMG amplitudes have been observed to occur during repetitive dynamically maximal and submaximal efforts, respectively [42].
Luttmann has proposed a new JASA approach to evaluate the fatigue caused by muscular activities [45]. Variations of EMG spectra and amplitudes throughout the activities are simultaneously considered in this method.
Therefore, JASA method provides the main advantage of the subjects’ uninterrupted isometric contractions to perform, for example, an MVC test. Our results suggested that, when fatigued, participants are in the direction of more dorsiflexion (or less plantarflexion), possibly indicating a protective mechanism to resist ankle sprain.
Because these changes were seen across plots, changes in JASA in patient group were likely due to neuromuscular compensations in response to fatigue rather than muscle weakness.
This study followed the relevant possible fatigue mechanisms at pushing up a load via the application of the JASA method.
Observing muscular fatigue is feasible through the decrease of the MDF throughout static contractions, even though the contraction force level is 80% MVC.
In the current research, muscular fatigue was felt in 80% of the recorded upper trapezius muscles during the push up activities versus the load after more than 3 minutes.
By applying the JASA method, researchers highlighted the muscular fatigue condition as well as the likely fundamental mechanisms.
The upper trapezius muscle kept the glenohumeral and scapulothoracic posture versus gravity through the work throughout the sustained shoulder elevation activity. Consequently, when the upper trapezius muscle feels a myofascial trigger point, it will experience more fatigue compared to the healthy one.
Roya Mehdikhani wrote the manuscript and designed the study. Olyaei Gholam Reza developed the original idea and did critical revision of the manuscript for important intellectual content. Talebian Moghadam Saeed studied concept and design. Hadian Mohammad Reza and Shadmehr Azadeh contributed.
The authors have no personal or financial relationships with other peoples or organizations that could present the potential conflict of interests in their works.
This study was supported by the School of Rehabilitation Tehran University of Medical Sciences, Tehran, Iran, as part of a Ph.D. thesis.
The authors would like to appreciate all individuals who took part in this research. The authors would like to appreciate the assistance of the faculty and the staff of Tehran University of Medical Sciences, the School of Rehabilitation, and I would like to thank Dr.Fatemeh Jafari, the head of the School of Allied Medical Sciences, for her encouragement.
Ethical clearance was obtained from the Tehran University of Medical Science Ethics Committee and was adhered to throughout the study.
Ethics Committee number IR.TUMS.VCR.REC 1395.803; 2016/10/22 and all subjects signed an informed consent form prior to their inclusion.
IRCT2017011426346N2
