 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Vargas Castillo Elvis1,2* , Pérez Mariangela1,2, Melo Amaral Ingrid2, Garcilazo Dimas1, Prados Manuel1
, Pérez Mariangela1,2, Melo Amaral Ingrid2, Garcilazo Dimas1, Prados Manuel1
1Department of Coloproctology, University Hospital Dexeus, Barcelona, Spain.
2Department of Coloproctology, Domingo Luciani Hospital, Caracas, Venezuela.
Correspondence to: Vargas Castillo Elvis, Department of Coloproctology, University Hospital Dexeus, Barcelona, Spain.
Received date: December 23, 2023; Accepted date: January 20, 2024; Published date: January 27, 2024
Citation: Elvis VC, Mariangela P, Ingrid MA, et al. Anorectal Melanoma: Not All Dark, Bleeding, and Painful Masses are Hemorrhoids. J Med Res Surg. 2024;5(1):6-9. doi: 10.52916/jmrs244127
Copyright: ©2024 Elvis VC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Anorectal Melanoma represents less than 1% of all gastrointestinal tumors. They are infrequent, aggressive and with little therapeutic consensus. The prognosis is usually reserved, with a five-year survival rate of less than 20%. The anorectal canal is the most common place where melanomas of the gastrointestinal mucosa appear. Thus, although it represents 0.05 to 4.6% of anorectal lesions, this constitutes the third most common location of melanoma, after the skin and eyes. Generally, their diagnosis is confusing and late, as they require a high index of suspicion; The symptoms are non-specific but they mostly present as dark, bleeding and painful masses, sometimes being confused with benign anorectal processes such as hemorrhoids. This occurred in two clinical cases that we presented in women aged 78 and 59 years with a purplish-black anorectal mass, the first case confused with hemorrhoidal thrombosis and the other with bleeding internal hemorrhoids; therefore, being treated as such in primary medical care centers and delaying their diagnosis by an average of 3 months (Figure A, B and E). Both cases with high clinical suspicion of a malignant process were later confirmed in a specialized unit, where they underwent biopsies and imaging studies. The patients underwent surgery, with subsequent chemo-immunotherapy. The first with abdomino-perineal resection (Figure C) plus inguinal lymphadenectomy (Figure E) and the other with local excision (Figure F). Both neo or adjuvant treatment and type of surgery remain controversial today.
Anorectal Melanoma (AM), Cutaneous Melanoma (CM), Benign anorectal process, Hemorrhoids, Delayed diagnosis, Controversial treatment.
Anorectal Melanoma (AM), initially described in 1857 by Moore, is a rare tumor with a very poor prognosis. The scientific evidence available on this neoplasia is heterogeneous and inconclusive. Furthermore, it is an entity that is difficult to diagnose clinically, due to very non-specific symptoms and because in a third of cases it presents as a non-pigmented lesion. Only a thorough examination, together with a high index of suspicion, will prevent diagnostic delay [1]. The epidemiological data on AM are very heterogeneous, it represents slightly less than 1% (0.1-4.6%) of anorectal malignancies and will account for approximately 1-2% of all melanomas. 90% of melanomas occur on the skin (Cutaneous Melanoma). The remaining 10% is divided between ocular melanoma (5%), melanoma of unknown origin (2%) and melanoma that occurs on the mucosa (3%). Within the latter, the AM represents the third most frequent location, after head/neck and female genital tract. However, among primary melanomas that occur in the gastrointestinal tract, the anorectal location is the most frequent, being somewhat more frequent in women, in a ratio of 1.5:1 [2]. The etiopathogenesis and risk factors for AM are poorly known, but there are certain differences with respect to ocular and cutaneous melanomas, such as environmental risks, for example: ultraviolet radiation clearly associated with CM, but not involved in the development of mucosal melanomas in general or anorectal melanomas in particular, a fact that could have significant implications from a therapeutic point of view [3]. With respect to histology, we could affirm that this does not seem identical to that of CM, although AM also has its origin in the malignant transformation of melanocytes, in this case, of the anal canal. These are organized into tumor niches that can be epithelioid (44%), mixed (31%) or spiculated (25%). Subsequently, these cells invade the squamous plane, expressing a series of immunospecific proteins for melanoma such as HMB-45, S-100 and vimentin. But in contrast to CM, a disproportionately high number of 10-87% according to most AM series will be "amelanotic." We do not know for sure whether or not this characteristic will have implications for the prognosis of the disease (beyond an obvious decrease in the index of suspicion), although there are st udies that show comparable survival results for both types of lesions. Perhaps the most relevant thing is to emphasize the fact that not all pigmented lesions of the anal canal are malignant melanomas, nor are all malignant melanomas pigmented [4].
The objective of this article is to present our experience with 2 patients in the diagnosis and treatment of anorectal melanoma and thus highlight the importance of a differential diagnosis with benign anal disease, especially with hemorrhoids, given the nodular and usually pigmented nature that they can present both injuries.
The first case is a 78-year-old woman who, 3 months before coming to our Unit, consulted a primary medical care center for presenting an anorectal nodular lesion, purplish in color, painful and bleeding for approximately 15 days. The initial diagnosis was hemorrhoidal thrombosis, which is why outpatient medical treatment with antihemorrhoidal creams, phlebotonics, laxatives and sitz baths was indicated. In view of the lack of improvement, she went again to other centers where the same diagnosis was based, but upon noticing rapid growth of the lesion, she was referred to our unit where, upon examination, the patient revealed a black tumor measuring approximately 8 cm in diameter violaceous, painful, bleeding, hard consistency, non-mobile and occludes the lumen of the anorectal canal by approximately 70% (Figure A,B). A biopsy is taken under local anesthesia in consultation and complementary studies are requested (blood tests, tumor markers, colonoscopy, Body CT, rectal MRI). The biopsy confirms the diagnosis and in the imaging studies a lesion measuring approximately 7 cm in its greatest diameter, occlusive, extends from the margin of the anus to the upper edge of the puborectalis muscle, infiltrates up to the mesorectal fat, with 3 lymph nodes. greater than 1 cm and adenopathy of approximately 2 cm in the left ilioinguinal chain suggestive of metastasis and without lesions in the liver, lungs or brain.
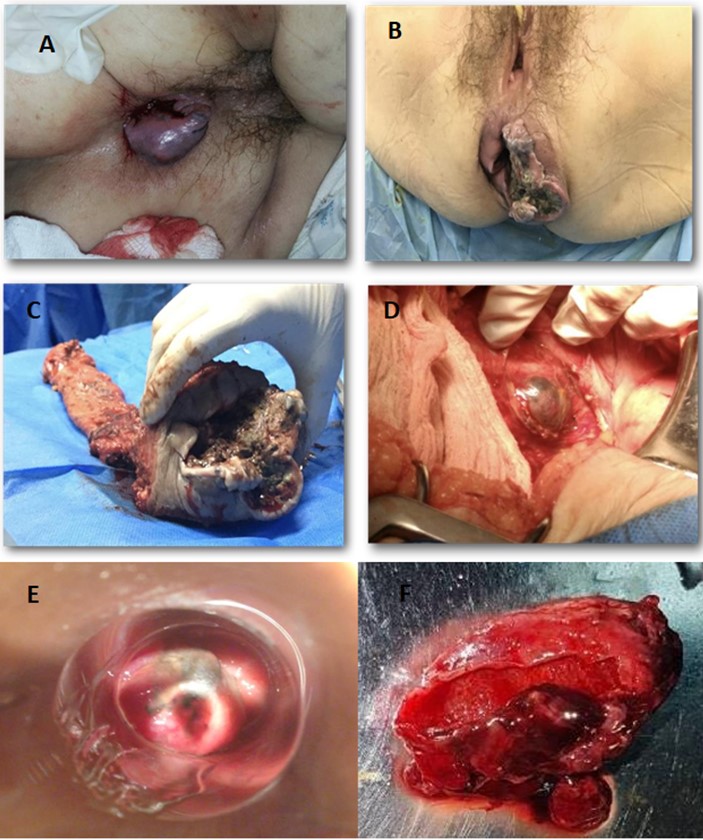 Figure 1: A) Anorectal melanoma confused with thrombosed haemorrhoid; B) At 2 months, with very rapid growth; C) Surgical specimen after abdominal perineal resection; D) with left inguinal lymphadenopathy in situ; E) Mucosal anorectal melanoma view by anoscopy, confused previously with bleeding haemorrhoid; F) Local excision.
Figure 1: A) Anorectal melanoma confused with thrombosed haemorrhoid; B) At 2 months, with very rapid growth; C) Surgical specimen after abdominal perineal resection; D) with left inguinal lymphadenopathy in situ; E) Mucosal anorectal melanoma view by anoscopy, confused previously with bleeding haemorrhoid; F) Local excision.The case is discussed in an oncological session and it is decided, in view of the patient's significant symptoms, to proceed to surgery and perform open Abdominoperineal Resection (APR) (Figure C) plus left ilioinguinal lymphadenectomy (Figure D), without incident. The definitive biopsy reports: poorly differentiated invasive malignant neoplasm measuring 7.5 cm with solid patterns, pseudoglandular and diffuse nests that infiltrate the muscularis propria to the mesorectal fat with evident lymphatic and perineural vascular permeability; of an exophytic and ulcerated stenosing annular configuration, which presents approximately 90% of cells with solid, diffuse patterns and in nests with a poor degree of differentiation, with foci of blackish-brown pigment; in less than 10% there are foci with glandular/pseudoglandular configuration, surgical edges free of lesion; 13 lymph nodes in total, 2 positive. The inguinal lymph node dissection revealed 3 lymph nodes, one of which was the largest with infiltration of neoplastic cells. In the presence of a high-risk melanoma, adjuvant treatment was proposed with 6 cycles of intravenous dacarbazine (250 mg/m2 of body surface area) for 5 days, every 3 weeks plus interferon, according to the Kirwood scheme, completing a total of 30 sessions, and then maintenance treatment with interferon, 15 million IU subcutaneously three times a week. After 3 years of follow-up, the patient presented unresectable lung and brain metastases and died.
The second case is a 59-year-old woman who is referred to our unit 15 days after receiving non-resolving treatment with a diagnosis of bleeding internal hemorrhoids. The proctological examination revealed a friable blackish-violet lesion measuring approximately 1.5 cm and located above the pectineal line (Figure E). A biopsy is taken in consultation that confirms the diagnosis of malignancy and complementary studies are performed (blood analysis with tumor markers, Colonoscopy, Body CT, Endoanal ultrasound). The analysis reveals mild anemia of 10.2 Hemoglobin, previous 13.3 and treatment with oral iron is started, Body CT without evidence of metastatic lesions and endoanal ultrasound with a lesion of approx. 1.8 cm that infiltrates up to the submucosa without penetrating the muscularis propria and without perirectal lymph nodes. The case is discussed and it is decided to perform transanal Local Excision (LE) (Figure F). The biopsy of the specimen reported spiculated melanoma with a maximum Breslow thickness of 5 mm. Likewise, infiltration of the rectal mucosa and submucosa, which reached 3 mm in depth. No perineural or angiolymphatic invasion and surgical margins free of neoplasia. Immunotherapy was started with interferon for 20 sessions and subsequent maintenance for 3 years. In his 5-year follow-up he is free of local and distant disease.
MA will present symptoms perfectly attributable to benign and much more common anorectal entities: rectal bleeding is the most frequent symptom, present in 53 to 96% of cases, followed by the presence of a nodule or mass sensation, tenesmus, proctalgia and, more sporadically, pruritus or change in bowel habit. This symptomatic courtship, seemingly banal, will last an average of 3-8 months until the definitive diagnosis and entails a rate of diagnostic errors close to 55% [5]. According to some series, the confusion of AM with hemorrhoidal disease has, in a statistically significant way, a negative impact on survival figures, as perhaps occurred in our first case. Anatomically, most anorectal melanomas are located in the anal canal or in the pectineal line. Only 2-5% settle exclusively in the rectal mucosa. They are usually tumors (pigmented or not) 1.9-3.8 cm in diameter, with an ulcerated, flat or polypoid appearance. A pigmented and polypoid lesion can be easily confused with a thrombosed hemorrhoid [6]. In fact, in a large review by the Memorial Sloan-Kettering Cancer Center (MSKCC), 8% of AM diagnoses were made after the anatomopathological study of hemorrhoidectomy specimens, which is why it is recommended, in a systematic way, to analyze all the resected pieces and send them to the pathologist identified topographically [7].
Thorough anuscopy and anal examination (accompanied by a good index of suspicion) are essential to determine the size, location and characteristics (fixation, pigmentation, depth, etc.) of the lesion. On the other hand, almost 20% of AM will present positive inguinal lymphadenopathy and 7-25% are diagnosed with distant metastases (generally bone, lung, liver or brain). This makes it important both to explore and study the inguinal region and to perform advanced imaging tests (pelvic MRI, CT and endoanal ultrasound) to help us make therapeutic decisions [8]. The PET-scan has a low sensitivity, so for the most part it is not recommended to perform it systematically, reserving it for doubtful lesions after the CT scan [9]. A controversial aspect is the thickness of the lesion, which according to some authors, is related to lymphatic dissemination, local recurrence after surgery and oncological results, providing survival figures of 33 months for tumors <4 mm thick, compared to 8 months. for those >4 mm. However, the literature is especially heterogeneous when addressing this issue, using classifications referring to CM, some already obsolete (such as Clark's), others more recent (Breslow) or not providing any data on the thickness of the lesion, which occurs , in more than half of the publications [10].
On the other hand, and given that, unlike CM, most AMs present a thickness of 4 mm at the time of diagnosis, the clinical and prognostic value of this parameter is debatable, which is why the classification of the American Joint Committee on Cancer for CM is not applicable to AM, and there is currently no validated system for the staging of anorectal melanomas, although some authors simplify their classification into the 3 classic stages (I: localized; II: spread to lymph nodes and III: distant metastasis) [11]. According to some studies, in AM, and unlike what happens with CM, it will be the depth of the tumor invasion, and not the thickness of the lesion, that determines the probability of lymphatic or distant dissemination and therefore, a review systematics proposes a new system that expands these 3 stages to 4, considering stage I if the tumor does not infiltrate the muscularis propria and II if it does not infiltrate it [6].
The classic surgical treatment of AM, Abdominoperineal Resection (APR) has never been prospectively validated. In fact, the high morbidity associated with this procedure and the perception that radical surgery did not obtain appreciable advantages in survival led to the questioning of the benefits of APR as an initial treatment option in AM. The first publication about this topic was in 1982 where Cooper et al. [12] after analyzing 227 patients, found similar survival rates for radical surgery or Local Excision. Since then, there have been multiple series, general reviews and some systematic reviews that provide similar conclusions, although to date there is no randomized, controlled trial that reliably demonstrates them. In 2012, Kanaan et al. [10] analyzed 21 publications, with almost 700 patients included, obtaining a similar mean survival for APR and LE (21 and 20 months, respectively). We must note, however, that there are series, such as the one carried out at MSKCC, that obtain greater survival for patients undergoing radical surgery, although this fact is not corroborated by an update of the results from the same institution [7].
It is important to clarify that the typical lymphatic spread of AM is unknown, with the possibility of spread to deep iliac, mesorectal or presacral nodes. There is also no evidence that performing a lymphadenectomy (inguinal, mesorectal or pelvic) leads to an improvement in survival [4]. Melanoma shows greater immunosusceptibility than tumors of other types and, therefore, has been the subject of a wide variety of trials based on immunotherapy, with some encouraging results, especially in advanced CM. However, in the specific case of AM, the use of immunotherapy (interferon, interleukins, vaccines, etc.) does not go beyond experimental therapy [13]. AM does not respond to chemotherapy. The most used drug, dacarbazine (or its oral analogue, temozolomide) has shown, both as monotherapy and in combination with other drugs, responses that are barely around 20%. The so-called biochemotherapy (combination of a biological agent with a conventional chemotherapy) has shown some usefulness in metastatic CM [14]. The use of radiotherapy in the treatment of AM is very controversial. Although melanoma has historically been a radioresistant tumor, some recent studies cast doubt on this attribution; Although the role of RT as palliative therapy has been established for 40-50% of patients with unresectable, recurrent or disseminated disease, who develop accompanying symptoms, such as bone pain, spinal cord compression, tumor hemorrhage or central nervous system dysfunction [15,16].
Anorectal melanoma is a disease that is very easy to confuse with benign anorectal diseases such as hemorrhoids, thus delaying its diagnosis. It is highly lethal and still has little therapeutic consensus. The figures are discouraging 80% of recurrences after surgery with curative intent, 12-15% survival at 5 years and 17-21 months of median survival (30 months for stage I and 22 months for stage II.
The authors have no conflicts of interest to report.
No.
