 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
Journal of Medical Research and Surgery
PROVIDES A UNIQUE PLATFORM TO PUBLISH ORIGINAL RESEARCH AND REMODEL THE KNOWLEDGE IN THE AREA OF MEDICAL AND SURGERY
 Indexed Articles
Indexed ArticlesSelect your language of interest to view the total content in your interested language
Humaira Haider Mahin1, Matthew Beck2, Toni Palasovski3 and Sarbar Napaki4
1Department of Surgery, Wollongong Hospital, NSW, Australia
2General and Breast Surgeon, Wollongong Hospital
3Oncoplastic Breast Surgery, Wollongong Hospital, NSW, Australia
4Department of Anatomical pathology, Wollongong Hospital, NSW, Australia
Correspondence to: Humaira Haider Mahin, Department of Surgery, Wollongong Hospital, NSW, Australia, Tel: +61452464512; E-mail: mahinhaider@gmail.com
Received date: March 4, 2020; Accepted date: March 11, 2020; Published date: March 18, 2020
Citation: Mahin HH, Beck M, Palasovski T, et al. (2020) An Uncommon Presentation of Li Fraumeni Syndrome. J Med Res Surg. 1(2): pp. 1-3.
Copyright: © 2020 Mahin HH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits
unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
We report a case of Li Fraumeni Syndrome (LFS), where our patient was affected by two malignancies before the age of 30: chondroblastic osteosarcoma and unilateral breast ductal carcinoma in situ. Her daughter was diagnosed with adrenocortical carcinoma at the age of four, and her mum was diagnosed with cervical cancer when she was in her 20’s. After her daughter’s cancer diagnosis, she and her family members underwent genetic testing. She and her daughter were found to have Tumour Protein 53 gene (TP53) mutation suggestive for Li Fraumeni Syndrome (LFS). Patients with LFS should be managed with a surveillance program with minimal exposure to radiation therapy due to their high risk for second primary cancer. This report will make the clinician aware of this syndrome for early diagnosis and management.
Li Fraumeni Syndrome (LFS), Breast cancer, Osteosarcoma, Surgery, Gene testing
A 22-year female was referred to our breast surgery department of a regional tertiary hospital, for risk-reducing bilateral mastectomy after it was discovered that she was a carrier of TP53 mutation or Li Fraumeni syndrome. Since bilateral mastectomy, reduces the risk of breast cancer by at least 90% in LFS patients. Following the diagnosis of adrenal cancer in her four-year-old daughter, our patient, her daughter, and her parents had TP53 gene mutation testing. Our patient and her daughter tested positive for TP53, where her parents were tested negative. It appeared the germline mutation had commenced with her. Five years ago, she was diagnosed with chondroblastic osteosarcoma of the left proximal femur which was managed with neoadjuvant chemotherapy, surgery and adjuvant chemotherapy in a specialized sarcoma unit after completion of her staging investigation revealed no CT evidence of metastatic pulmonary disease and no evidence of disseminated disease on PET. In her family, there were multiple members with cancer her mother had cervical cancer in her 20’s and breast cancer at her 50’s, and maternal uncle had lung cancer. On examination, she had grade 1 ptosis in C cup size breasts. There was no nipple change. There were no cutaneous manifestations of breast disease. There were no palpable lumps in either breast with no axillary adenopathy. Her examination overall was benign. Her bilateral mammogram, including tomosynthesis, showed heterogeneously dense breasts. There was no stellate mass, architectural distortion or suspicious microcalcification. On the three-dimensional images, there were no suspicious findings. Her bilateral breast ultrasound showed no solid or cystic lesions. There was no axillary lymphadenopathy. Multiplanar multiaxial breast MRI imaging was performed including pre-Gadoliniumand post-Gadolinium T1 as well as T2 weighted images. There was no concerning mass, distortion or atypical enhancement noted. No abnormal axillary lymph node or intramammary lymph node was seen (Figure 1).
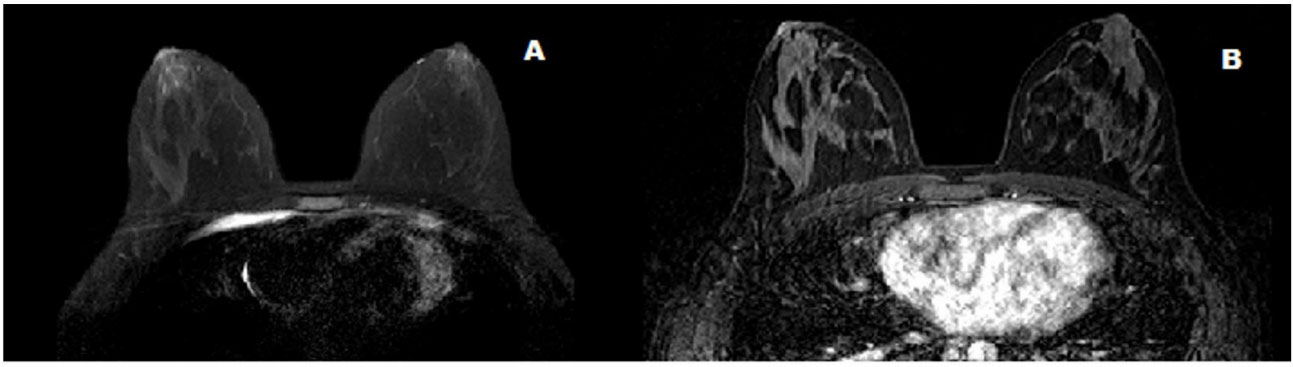 Figure 1: Magnetic resonance imaging (MRI) of bilateral breasts (axial) A: uniform fat suppression B: no concerning lesion on dynamic contrast-enhancing MRI.
Figure 1: Magnetic resonance imaging (MRI) of bilateral breasts (axial) A: uniform fat suppression B: no concerning lesion on dynamic contrast-enhancing MRI.Screening for metastatic disease by computed tomography and positron emission tomography showed no evidence of adjacent lymphatic, pulmonary bone or hepatic metastases. So, we did a bilateral nipple-sparing mastectomy with immediate reconstruction for her. By doing bilateral mastectomy, on histopathology, on the right breast, sections taken from the inner upper quadrant and central breast showed high- grade ductal carcinoma in-situ which is 70mm in maximum dimension and solid and focally cribriform in architecture. (Figure 2).
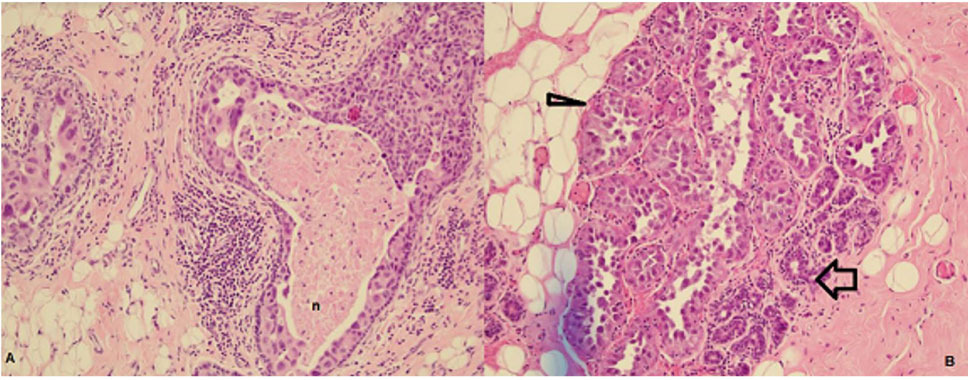 Figure 2: A): High grade DCIS with necrosis (n), B): Partially cancerised lobule: Normal tubules marked with a wide arrow, abnormal expanded large
tubules with atypical cells marked with a narrow triangle
.
Figure 2: A): High grade DCIS with necrosis (n), B): Partially cancerised lobule: Normal tubules marked with a wide arrow, abnormal expanded large
tubules with atypical cells marked with a narrow triangle
.There was no calcification. The resections margins were clear by more than 10mm. Immunohistochemical analysis showed expression of Estrogen receptors 50%, progesterone receptors focal, Human Epidermal growth factor Receptor 2 (HER2) classified as strong positive- 3+ (Figure 3) (I.e. intense cytoplasmic membrane staining in at least 30% of tumour cells) and P53 positive. Only focal areas show more well-defined changes involving multiple ducts while other areas show some cells having high-grade cytomorphology. No macroscopic lesion was seen. There was no evidence of ductal carcinoma in situ in those sections taken from the left breast Her BRCA1 and BRCA2 gene mutation analysis were negative.
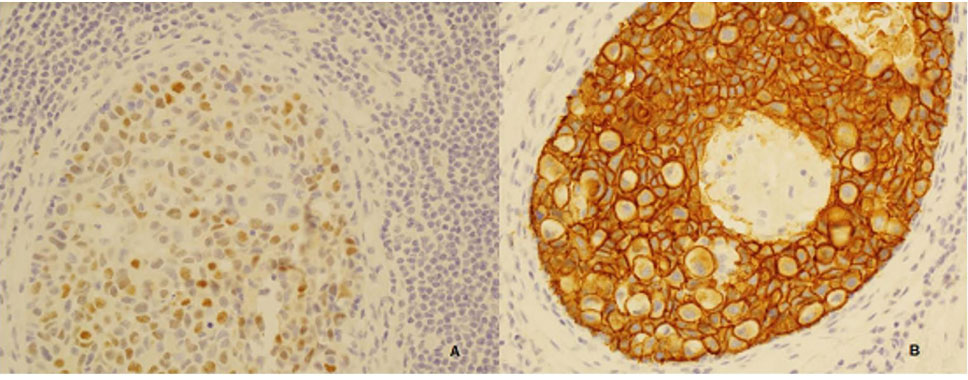 Figure 3: Immune- histochemical analysis: immune peroxidase stain, A): brown staining nucleus suggestive of Estrogen receptor-positive, B): Circumferential membrane with complete membrane staining in 100% of cells suggestive of strong HER2 positive status.
Figure 3: Immune- histochemical analysis: immune peroxidase stain, A): brown staining nucleus suggestive of Estrogen receptor-positive, B): Circumferential membrane with complete membrane staining in 100% of cells suggestive of strong HER2 positive status.Support from multiple subspecialties, e.g. clinical geneticist, genetic counsellor, surgical team, oncological team, and psychologist had been organised for her. She was also discussed at our specialised multidisciplinary meeting and was suggested for a whole-body bone scan and a whole-body MRI scan accepting the artifact from pelvic prosthesis and breasts implant. She will have dedicated breast and brain MRI scan. Yearly follow-up with dermatologist for skin check for melanoma had been organised. She did not need any further chemotherapy. She was advised to have a faecal occult blood test done annually. She was enrolled in the ‘The Surveillance study in Multi-Organ Cancer prone syndromes’ (SMOC) [1] for screening or early detectionof any other primary cancer. She is currently well without any findings of cancer recurrence.
LFS is an inherited autosomal dominant disorder that is expressed by a distinct range of malignancies [2]. The lifetime risk of cancer in LFS patients is extraordinarily high, with 50% of patients having a diagnosis of cancer by the age of 30% and 90% by the age of 50 [3]. The tumour protein p53 gene (TP53) has a close relation with LFS. To verify the destiny of cells which have damaged DNA, TP53 plays a vital role by delaying the cell cycle progression, allowing DNA repair or starting apoptosis. In the case of mutation of TP53 gene mutation, the cells containing damaged DNA can continue to proliferate and promote malignant transformation. TP53 mutation identified in our patient and her daughter was not identified in our patient’s mother or father. This means there is a new mutation in our patient and her daughter inherited this abnormal TP53 gene as an autosomal dominant pattern.
Women with LFS are eighteen times more likely to develop breast cancer before their forty-fifth birthday compared with the general population [4]. Other reported second primary tumour associated with LFS are brain cancer (20-30% by age 70), adrenocortical carcinoma, soft tissue sarcoma (50% by the age of 70 years), colorectal cancer (24%) and gastric cancer (1- 16%) [4]. LFS patients have increased risk and, or earlier onset of other second primary tumours such as lung, renal, melanoma and pancreatic cancer. [5] It has been reported that radiation therapy-induced cancers are more common in patients with LFS [5]. Our patient did not receive any radiation therapy in the past. For breast cancer in LFS, it usually has enhanced human epidermal growth factor receptor 2 (HER2). On immunocytochemistry study, our patient showed strong HER2 positive, score 3+ since the complete, strong membrane staining was noted on >30% of cells. The other commonly reported causes of early onset of breast cancer are BRCA1 and BRCA2 mutations. However, our patient tested negative for both BRCA1 and BRCA2 mutations.
BRCA1 mutations are strongly associated with triple-negative breast cancer, i.e. there is no expression of estrogen receptor, progesterone receptor and HER2 receptor [2]. Our patient was triple-positive breast cancer. Our patient was a perfect example of Classical LFS since a sarcoma was diagnosed before age 45 years and she had a first degree relative with any cancer before age 45 years, and she had a first degree relative with any cancer before age 45 years. She was suitable for genetic counseling and TP53 gene mutation testing.
These are the other high-risk conditions when we should consider TP53 mutation testing: early onset of breast cancer (less than age 31 years) without detectable BRCA1 or BRCA2 gene mutation, family history of sarcoma, brain tumour or adrenocortical tumour. A thorough screening process should run for the patients who have the above criteria but negative for detectable TP53 gene mutation and for patients who decide not to have this mutation testing. Furthermore, the negative result for TP53 gene mutation does not rule out LFS. We also suggest routine TP53 gene testing in early-onset breast cancer (diagnosed before the age of 30) patients who have strong HER2 positive status and family history of a range of cancers.
In conclusion, we are presenting this case of LFS to make clinicians aware that genetic testing should be considered while managing young sarcoma patients with multiple cancer positive family history for early diagnosis of other second primary cancer and to influence care for their family members.
The authors declare no financial or non- financial conflicts of interest concerning the research, authorship, and publication of this article.
