 Journal of Clinical and Biomedical Investigation
PROVIDES A UNIQUE PLATFORM COVERING SCIENTIFIC KNOWLEDGE IN BIOMEDICAL SCIENCES AND CLINICAL RESEARCH
Journal of Clinical and Biomedical Investigation
PROVIDES A UNIQUE PLATFORM COVERING SCIENTIFIC KNOWLEDGE IN BIOMEDICAL SCIENCES AND CLINICAL RESEARCH
 Journal of Clinical and Biomedical Investigation
PROVIDES A UNIQUE PLATFORM COVERING SCIENTIFIC KNOWLEDGE IN BIOMEDICAL SCIENCES AND CLINICAL RESEARCH
Journal of Clinical and Biomedical Investigation
PROVIDES A UNIQUE PLATFORM COVERING SCIENTIFIC KNOWLEDGE IN BIOMEDICAL SCIENCES AND CLINICAL RESEARCH
Journal of Clinical and Biomedical Investigation brings articles in the areas related to all the fields of clinical and biomedical research on biannualy basis.
As opposed to the traditional subscription-based models, open access allows readers to access, use, and share academic work freely and with no charge. This allows for significantly more engagement and provides authors with a stronger platform to share their knowledge and the findings of their research. Since open access grants users’ restrictions-free access to high-quality materials, we have at our disposal a larger array of marketing and promotional tools to expand our readership than traditional publishers typically do. Anyone with a smartphone or a computer and access to the internet will be able to read your manuscript and share it with their peers.
Open access does not mean that authors give up control over their work. In fact, with open access authors are able to maintain the copyright, and readers are required to follow a set of specific rules on how they can correctly use and distribute this work. All work is protected by the Creative Commons Attribution License. The authors' publications in JCBI are distributed under the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/ ). The license was developed to facilitate open access, namely, free immediate access to and unrestricted reuse of original works of all types.
Under this license, authors retain ownership of the copyright for their publications but grant JCBI a non-exclusive license to publish the work in paper form and allow anyone to reuse, distribute and reproduce the content as long as the original work is properly cited.
Appropriate attribution can be provided by simply citing the original work. No permission is required from the authors or the publishers. For any reuse or distribution of a work, users must also make clear the license terms under which the work was published.
The standard license will be applied to the authors' publications, which ensures the publications freely and openly available in perpetuity.
Journal of Clinical and Biomedical Investigation believes that peer-review process has an important role in authenticating research works in science, health and medicine before final publication. It also helps in analyzing whether the submitted manuscript is relevant to the scope of the journal. Peer Review Process assists in maintaining Journal ethical standards and safeguards the truthful honest research works. The journal follows a double-blind peer review process. In double-blind peer review system, the identity of both the author and reviewer is kept hidden and our expert reviewers provide comments on the quality and content of the submitted articles on the discoveries and current novel developments in the mode of original articles, review articles, case reports, short communications, etc. while making them freely available through online without any restrictions.
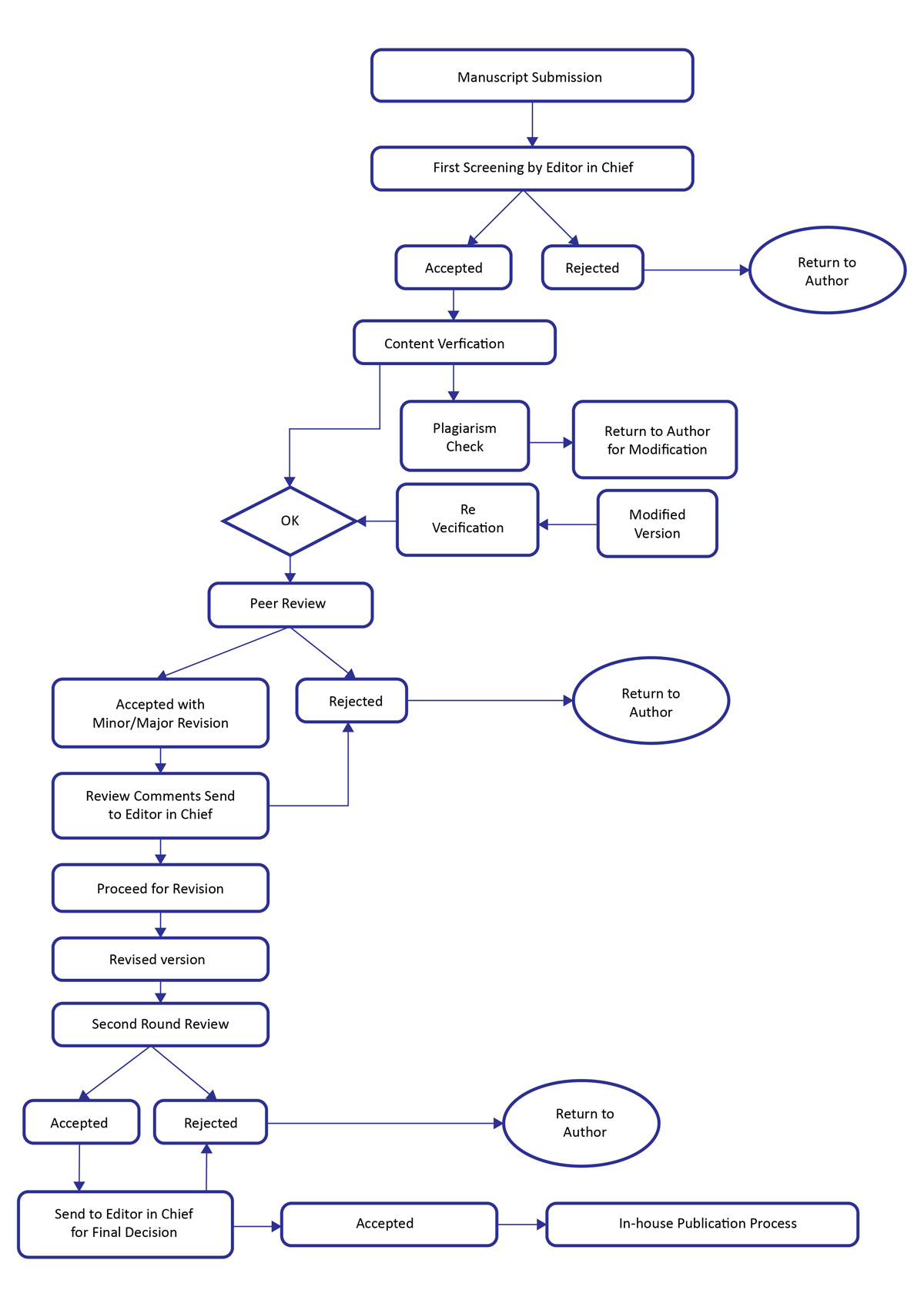
The single most important criterion for acceptance for publication is the originality of the work. Other factors may affect decisions, such factors are, the extent and importance of new information in the paper compared with that in other papers being considered.
The main mechanisms for ensuring the scientific quality of published articles are peer-review, editorial scrutiny. The submitted articles are rigorously peer-reviewed to ensure to accept the high quality submissions, and the quality is controlled by the Editor(s)-in-Chief or editorial board members. The editorial board members and peer reviewers are all experts in their field from various countries and regions around the world. The published articles reflect the up-to-date research findings, with reliable results, objective and un-biased discussion of the results. In the production stage, the copyediting, layout, proofreading, and online publication are all maintained in highest possible quality.
Submission to Journal of Clinical and Biomedical Investigation is taken by the journal to mean that all the listed authors have agreed all of the contents, including the author guidelines and author contributions statements. Before submission, the corresponding author ensures that all authors are included in the author list, its order has been agreed by all authors, and that all authors are aware that the paper was submitted. The corresponding author is solely responsible for communicating between the journal and all co-authors, during the manuscript submission, peer review, and publication process.
Authorship needs to be justified by describing the role and contribution of each author on the title page of article as it implies responsibility and accountability for published work.
Authorship roles can vary upon significant intellectual contribution, responsibility and accountability for published work. So, authors sequence and correspondence should be determined by the participants early in the research process, to prevent disputes and misunderstandings. When an author discovers a significant error or inaccuracy in his/her own published work, it is the author's obligation to promptly notify the journal editor or publisher and cooperate with the editor to retract or correct the paper. The journal will not necessarily correct errors after publication if they result from errors that were present on a proof that was not shown to co-authors before publication. The corresponding author is responsible for the accuracy of all content in the proof, in particular that names of co-authors are present and correctly spelled, and that addresses and affiliations are current.
Authors reporting results of original research should present an accurate account of the work performed as well as an objective discussion of its significance. Underlying data should be represented accurately in the manuscript. A paper should contain sufficient detail and references to permit others to replicate the work. Fraudulent or knowingly inaccurate statements constitute unethical behavior and are unacceptable.
The authors should ensure that they have written entirely original works, and if the authors have used the work and/or words of others that this has been appropriately cited or quoted. Multiple, redundant or concurrent publication an author should not in general publish manuscripts describing essentially the same research in more than one journal or primary publication. Parallel submission of the same manuscript to more than one journal constitutes unethical publishing behavior and is unacceptable. Proper acknowledgment of the work of others must always be given. Authors should also cite publications that have been influential in determining the nature of the reported work.
When an author discovers a significant error or inaccuracy in his/her own published work, it is the author’s obligation to promptly notify the journal’s Editor or publisher and cooperate with them to either retract the paper or to publish an appropriate erratum.
Journal of Clinical and Biomedical Investigation observes ethics in publication process on the basis of guidelines issued by International Committee of Medical Journal Editors (ICMJE). Journal of Clinical and Biomedical Investigation works with the mission of non-discriminatory publication and expects the same from the authors, editors and reviewers. The authors must include an ethics statement describing the details of the committees who approved their experiments. The ethics statement must also confirm that informed consent was obtained from all the recipients. At the time of peer-review, editors may request ethical statement or informed consent letters.
Journal of Clinical and Biomedical Investigation expects that authors present accurate, original and objective research in the form of manuscripts. The authors are expected to preserve the raw data and any other valuable information related to the research. The editorial board may review the raw data in relation to the manuscript under publication consideration. The authors are expected to cite properly the publications that have been influential in determining the nature of the reported work. The authors are advised not to publish same manuscripts in more than one journal at the same time. Submitting the same manuscript to more than one Journal or publication will be considered unethical. Such behaviour is convicting and unacceptable. It is the responsibility of the authors to promptly notify editors about the errors and cooperate with them to withdraw or correct the submitted manuscript.
Editors play an important role in scholarly publishing Journal’s. JCBI accept prominent personalities in various research fields, who could carry out their responsibilities with much dedication to improve the quality of the Journal. The editors will be accountable for evaluating the quality quotient of the articles submitted for publication in Journal of Clinical and Biomedical Investigation. The editors should ensure that the articles are evaluated according to journal guidelines and constructive feedback is provided to the authors in order to enhance the quality of the articles.
The editors are expected not to disclose any information about a submitted manuscript to anyone other than the corresponding author(s), reviewers and other concerned members of the editorial board.
The editors should ensure that they complete the review process within stipulated time so that manuscripts are processed and reach the publication stage on fast track basis. The consistency in publication will also enforce a feeling of trust among the authors.
Guidelines to be followed
Confidentiality: Reviewers should not share any information from an assigned manuscript with outsiders without the prior permission from the Editor or preserve the data from an assigned manuscript.
Competence: Reviewer with fair expertise should complete the review. Assigned Reviewer with inadequate expertise should feel responsible and may decline the review as it is presumed that reviewer will be an expert in the respective field.
Constructive assessment: Reviewer comments should appreciate positive aspects of the work, identify negative aspects constructively, and indicate the enhancement needed. A reviewer should explain and support his or her judgment clearly enough that Editors and Authors can understand the basis of the comments. The reviewer should ensure that an observation or argument that has been previously reported be accompanied by a relevant citation and should immediately alert the Editor when he or she becomes aware of duplicate publication. A reviewer should not use any kind of abusive language while commenting on an article. Judgment of each article should be done without any bias and personal interest by the assigned reviewer.
Impartiality and Integrity: Reviewer’s decision should solely depend on scientific merit, relevance to the subject, scope of the journal rather than financial, racial, ethnic origin etc., of the authors.
Disclosure of conflict of interest: To the extent feasible, the reviewer should minimize the conflict of interest. In such situation, reviewer should notify the editor describing the conflict of interest.
Timeliness and responsiveness: Reviewers should morally abide to provide the review comments within the stipulated time and be active enough in responding to the queries raised by the editor if any.
All authors should disclose in their manuscript any financial or other substantive conflicts of interest that might be construed to influence the results or their interpretation in the manuscript. All sources of financial support for the project should be disclosed.
It is mandatory for the authors reporting experiments with the involvement of human and animal subjects to adhere to following facets:
The authors should include a statement in the article that they have identified the related institutional and/or licensing committee and have taken approval from the committee for their experiments. They should also mention the address of the committee.
The authors should declare that they have conducted all experiments in accordance with relevant guidelines and regulations of the committee.
If an author performs experimental studies involving client-owned animals, he/she must present an acceptance document from the client about best practices in animal testing. Field studies and other non-experimental research on animals must observe institutional, national, or international guidelines, and wherever available should have been approved by an appropriate ethics committee.
The authors involved in research and experiments involving human subjects must confirm that informed consent was obtained from all participants and/or their legal guardians.
The U.S. Public Health Service's Policy on Humane Care and Use of Laboratory Animals (PHS policy) and the Guide for the Care and Use of Laboratory Animals describe general policies and procedures designed to ensure the humane and appropriate use of live vertebrate animals in all forms of medical research. Journal of Clinical and Biomedical Investigation finds the policies and procedures set forth in the PHS policy and the Guide for the Care and Use of Laboratory Animals to be both necessary and sufficient to ensure a high standard of animal care. The researchers are expected to conduct their animal research in compliance with PHS policy.
The Editor-In-Chief will evaluate the submitted manuscripts on animal well-being issues and if the research found to be inconsistent with commonly accepted norms of using the animal and human subjects, the manuscript is liable to be rejected. The Editor-In-Chief also reserve the right to contact the approving committee for any further clarification. Researchers please note that they have a moral obligation towards the animals and human subjects they use for their research goals and they must treat them with compassion and consider their well-being while designing the projects.
 Plagiarism, duplicate submission, data falsification, inappropriate authorship credit, and all the like misconducts are not tolerated. This journal uses PlagScan Software to detect any possible plagiarism in the submissions.
Plagiarism, duplicate submission, data falsification, inappropriate authorship credit, and all the like misconducts are not tolerated. This journal uses PlagScan Software to detect any possible plagiarism in the submissions.
Informed consent is required for any paper to be published in Journal of Clinical and Biomedical Investigation if that research involves human participants. Informed Consent policy states that a participant in research must be informed about all aspects of the trial and the research should be carried out only when the participant voluntarily confirms his or her willingness to participate in a particular clinical trial and significance of the research for advancement of medical knowledge and social welfare.
In case report(s), the authors should include a statement that the patient's consent has been obtained if the patient's identifying information, such as name, hospital number, and any other privacy nature items will be published due to the information are essential for the scientific purposes. In the manuscript, there should be such statement "Patient's informed consent for publication of this report was obtained". If there is not any identifying information in the manuscript, authors may state "Informed Consent: Not Applicable".
In most clinical studies (trials), retrospective or prospective nature, with human subjects involved, the individual patient informed consent is required; there should be such statement in the manuscript. If such informed consent is not necessary or exempted judged by the Ethical Committee (such as in some retrospective studies), the authors should clearly state such waiver of informed consent in the manuscript.
If the patient is deceased, the authors should seek informed consent from a relative of the patient. If neither the patient nor a relative can provide such consent, the manuscript can only be accepted and published if the identifying information has been sufficiently anonymized and covered.
In particular, please remove any identifying information (patient name, birth, hospital number, etc) in the images, ECG report, pathology slides, X-ray, ultrasound and any other imaging data prior to submission, if these information are not relevant to scientific purposes.
The authors should keep the signed informed consent form; a copy should be available upon journal editor's request.
Journal of Clinical and Biomedical Investigation completely follows the regulations from ICMJE. Also, Journal of Clinical and Biomedical Investigation is a member of ICMJE at present.
The manuscript is thoroughly checked by the peer-review council after the author sends the revised manuscript, to ensure that recommended suggestions are implemented in the article content. Thereafter, Galley proofs are sent to the author for proofreading and corrections are done before the PDF's are sent for final publication.
After final publication:Once the article is finally published, changes with regard to author names, affiliations etc. will not be entertained. However, if any mistake is detected that seriously affects the publication records or the scope of the article then modifications can be done on receipt of such request from the corresponding author of the article.
Policies related to retraction of the articles covers instances leading to unethical practices in developing an article, multiple submission, fake authorship, and any other fraudulent conduct. It also covers requests to retract an article for correcting mistakes and technical errors in the research data. We adopt to the best practices for dealing with retractions and allow genuine requests on receiving such requests with a retraction request letter duly signed by the signed by the authors. After approval, the HTML and other versions of the article are removed from the Journal of Clinical and Biomedical Investigation database and other promotional platforms.
Manuscripts may be withdrawn by submitting a letter to the editorial office stating the reasons for manuscript withdrawal. There are no withdrawal charges if the manuscript is withdrawn before plagiarism check but the authors need to pay 50% of the publication fee as Withdrawal charges after 7 days of the manuscript submission.
Replacement of the article is accepted in cases where the article, if acted upon, might pose a serious health risk and the authors of the original article wish to retract the original and replace it with a corrected version.
The above policies are designed and implemented to address any concern of the authors and to take into account best practice in the scholarly publishing. These policies are revisited on scheduled basis and revised as per the international standards and best practices adopted by the publishing and information industry.
