 Journal of Clinical and Biomedical Investigation
PROVIDES A UNIQUE PLATFORM COVERING SCIENTIFIC KNOWLEDGE IN BIOMEDICAL SCIENCES AND CLINICAL RESEARCH
Journal of Clinical and Biomedical Investigation
PROVIDES A UNIQUE PLATFORM COVERING SCIENTIFIC KNOWLEDGE IN BIOMEDICAL SCIENCES AND CLINICAL RESEARCH
 Journal of Clinical and Biomedical Investigation
PROVIDES A UNIQUE PLATFORM COVERING SCIENTIFIC KNOWLEDGE IN BIOMEDICAL SCIENCES AND CLINICAL RESEARCH
Journal of Clinical and Biomedical Investigation
PROVIDES A UNIQUE PLATFORM COVERING SCIENTIFIC KNOWLEDGE IN BIOMEDICAL SCIENCES AND CLINICAL RESEARCH
Anubha Bajaj *
Consultant Histopathologist, AB Diagnostics, India.
Correspondence to: Anubha Bajaj, Consultant Histopathologist, AB Diagnostics, India.
Received date: January 13, 2023; Accepted date: January 24, 2023; Published date: January 28, 2023
Citation: Bajaj A. The Versicolour Gyre-Squamous Cell Carcinoma-Anal Canal: A Mini Review. J Clin Biomed Invest. 2023;3(1): 1-3. doi:
10.52916/jcbi234021
Copyright: ©2023 Bajaj A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits
unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Squamous cell carcinoma of anal canal is a frequently discerned malignant neoplasm confined to the anal tract. Thus denominated by World Health Organization(WHO), the neoplasm exemplifies primary squamous cell carcinoma of anus. Basaloid variant of squamous cell carcinoma is additionally designated as cloacogenic or transitional cell carcinoma of anal canal. Besides, mucoepidermoid carcinoma of anal canal denominates neoplasms delineating prominent features of mucinous transition.
Squamous Cell Carcinoma (SCC), Anal Canal, Cancer cells, Tumour
Squamous cell carcinoma of anal canal is a frequently discerned malignant neoplasm confined to the anal tract. Thus denominated by World Health Organization (WHO), the neoplasm exemplifies primary squamous cell carcinoma of anus. Basaloid variant of squamous cell carcinoma is additionally designated as cloacogenic or transitional cell carcinoma of anal canal. Besides, mucoepidermoid carcinoma of anal canal denominates neoplasms delineating prominent features of mucinous transition. Squamous cell carcinoma of anal canal arising superior to dentate line are generally discerned within sixth decade and depict a female preponderance. Squamous cell carcinoma of anal canal subjacent to dentate line are commonly exemplified within third decade and demonstrate a male predominance [1,2]. Squamous cell carcinoma of anal canal emerging subjacent to dentate line is commonly associated with definitive conditions as a condyloma, anal fistula or exposure to radiation. The neoplasm may be engendered on account of exposure to high risk subtypes of Human Papilloma Virus (HPV) as 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 or 68. Frequently, tumefaction exhibits overexpression of Epidermal Growth Factor Receptor (EGFR) [2,3]. Squamous cell carcinoma of anal canal may represent with cogent clinical symptoms as rectal bleeding, pain or mass within anal region. Upon gross examination, squamous cell carcinoma of anal canal manifests as a nodular, ulcerated neoplasm with magnitude varying from ≥3 centimetres to 4 centimetres. Tumefaction is associated with deep seated infiltration into anal canal wall with proximal and distal tumour extension into submucosa of distal rectum and proximal anus [3,4].
Grading of squamous cell carcinoma is contingent to actors such as nuclear pleomorphism, nucleolar magnitude, mitotic activity and tumour associated necrosis. Squamous cell carcinoma is graded as:Squamous cell carcinoma of anal canal exemplifies cogent morphologic variants denominated as:
Basaloid subtype demonstrates a plexiform pattern of tumour configuration with palisading of miniature undifferentiated cells circumscribing tumour cell nests or nodules with foci of centric necrosis. Mitotic figures are frequent. Encompassing stroma appears desmoplastic. Tumour invasion into circumscribing soft tissue is commonly discerned. The variant enunciates an aggressive biological behaviour. Nodules of neoplastic squamous epithelial cells may exemplify significant infiltration of eosinophils, foci of mucoepidermoid carcinoma with configuration of mucinous micro-cysts or appear as a poorly differentiated tumefaction [3,4]. Squamous cell carcinoma of anal canal may enunciate ‘small cell’ morphology with definitive features of anaplasia. However, foci of neuroendocrine differentiation are absent. Frequently, foci of squamous cell carcinoma of anal canal replace crypts confined within adjacent rectal mucosa. The neoplasm may delineate dysplastic alterations within superimposed or adjacent stratified squamous epithelium, thereby articulating anal intraepithelial neoplasia [3,4].
TNM classification of squamous cell carcinoma of anal canal is designated as:
Direct tumour invasion of rectal wall, perirectal cutaneous surface, subcutaneous tissue or sphincter muscles does not classify as T4. Tumour magnitude may be evaluated upon diverse planes wherein longest tumour diameter requires assessment.
Squamous cell carcinoma of anal canal is graded as
Staging of squamous cell carcinoma of anal canal is designated as
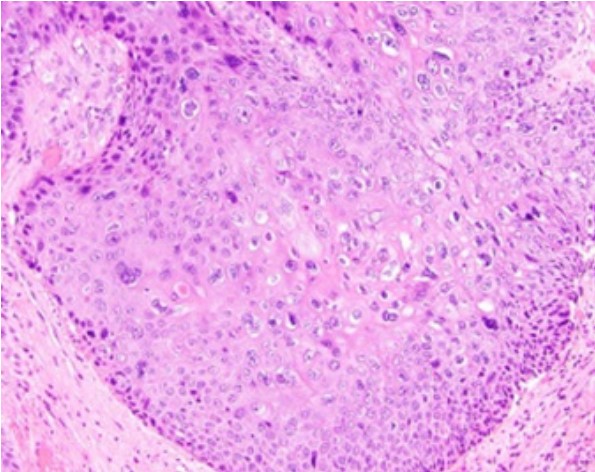 Figure 1: Squamous cell carcinoma of anal canal demonstrating enlarged
squamous cells with abundant eosinophilic cytoplasm, coarse nuclear
chromatin and prominent nucleoli. Intercellular bridges and mitotic figures
are discernible. Keratin pearls are absent [7].
Figure 1: Squamous cell carcinoma of anal canal demonstrating enlarged
squamous cells with abundant eosinophilic cytoplasm, coarse nuclear
chromatin and prominent nucleoli. Intercellular bridges and mitotic figures
are discernible. Keratin pearls are absent [7].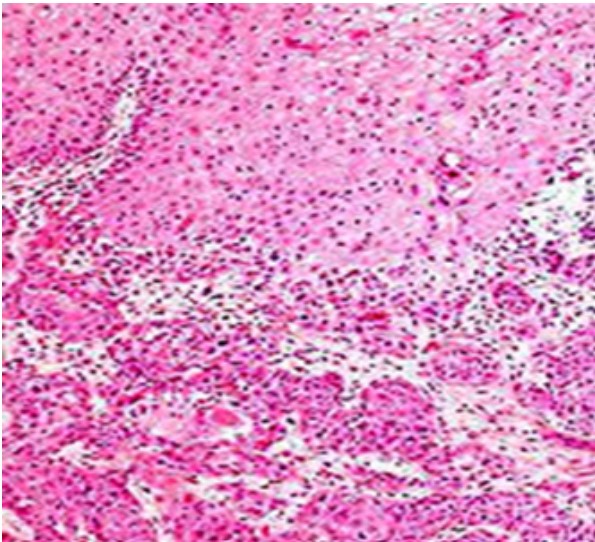 Figure 2: Squamous cell carcinoma of anal canal delineating enlarged
squamous cells imbued with abundant eosinophilic cytoplasm, vesicular
nuclei with conspicuous nucleoli and intercellular bridges. Focal necrosis
and reactive inflammatory infiltrate within circumscribing desmoplastic
stroma is encountered [8].
Figure 2: Squamous cell carcinoma of anal canal delineating enlarged
squamous cells imbued with abundant eosinophilic cytoplasm, vesicular
nuclei with conspicuous nucleoli and intercellular bridges. Focal necrosis
and reactive inflammatory infiltrate within circumscribing desmoplastic
stroma is encountered [8].Squamous cell carcinoma of the anal canal requires segregation from neoplasms such as basal cell carcinoma, small cell carcinoma, verrucous carcinoma or conditions such as anal fissure, anal fistula, haemorrhoids, psoriasis or condylomata acuminatum. Squamous cell carcinoma of anal canal can be appropriately treated with cogent surgical eradication of neoplasm followed by adjuvant chemotherapy and radiation therapy [5,6]. Prognostic outcomes of squamous cell carcinoma of anal canal are contingent to tumour stage as assessed with American Joint Committee of Cancer (AJCC) guidelines. Neoplasms situated upon distal sites appear amenable to preliminary detection, demonstrate gradual tumour progression and superior prognostic outcomes. Prognosis is favourable in neoplasms treated with enhanced dose of radiation therapy administered continually or with minimal interruptions. Prognosis is unfavourable in neoplasms incriminating elderly male subjects or individuals infected with Human Immunodeficiency Virus (HIV) [5,6].
