 Journal of Obstetrics and Gynecological Surgery
A PLATFORM FOR SCIENTIFIC INQUIRY IN THE FIELD OF OBSTETRIC AND GYNECOLOGY FIELD
Journal of Obstetrics and Gynecological Surgery
A PLATFORM FOR SCIENTIFIC INQUIRY IN THE FIELD OF OBSTETRIC AND GYNECOLOGY FIELD
 Journal of Obstetrics and Gynecological Surgery
A PLATFORM FOR SCIENTIFIC INQUIRY IN THE FIELD OF OBSTETRIC AND GYNECOLOGY FIELD
Journal of Obstetrics and Gynecological Surgery
A PLATFORM FOR SCIENTIFIC INQUIRY IN THE FIELD OF OBSTETRIC AND GYNECOLOGY FIELD
Anubha Bajaj*
Consultant Histopathologist, AB Diagnostics, India
Correspondence to: Anubha Bajaj, Consultant Histopathologist, AB Diagnostics, India.
Received date: July 3, 2022; Accepted date: July 13, 2022; Published date: July 20, 2022
Citation: Bajaj A. The Mellowed Trinitarian Mature Teratoma Ovary: A Short Communication. J Obst Gynecol Surg. 2022;3(2):16-17. doi:10.52916/jogs224026
Copyright: ©2022 Bajaj A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use,
distribution and reproduction in any medium, provided the original author and source are credited.
Neoplasm, Mature teratoma, Metamorphosis, Debris, Anechoic sebum, Surgical eradication
Mature teratoma is a frequently discerned, benign, ovarian germ cell neoplasm comprised of mature tissue manifesting two or three embryonic layers as ectoderm, mesoderm or endoderm. Additionally designated as mature cystic teratoma, mature solid teratoma or dermoid cyst, mature tissue minimally emerging from two embryonic layers is necessitated for cogent disease discernment. Malignant metamorphosis may ensue wherein prognostic outcomes are contingent to subtype of malignancy. Mature teratoma commonly occurs within reproductive age group. An estimated 10% neoplasms are bilateral. Mature teratoma is a diploid tumour and enunciates a normal, 46XX karyotype [1,2].
Mature teratoma is posited to arise from primordial germ cells. Although contributory factors remain unexplained, tumefaction is often discerned as an incidental finding. The gradually progressive neoplasm may exceptionally manifest features such as tumour rupture or ovarian torsion. Mature teratoma may be associated with conditions such as autoimmune haemolytic anaemia, paraneoplastic encephalitis or mucinous ovarian neoplasms as mucinous cystadenoma. Upon gross examination, a globular cyst with smooth extraneous surface of <10 centimetre magnitude may be delineated. Cut surface exhibits a cystic cavity imbued with hair, teeth, cartilage, bone or sebaceous material. Cyst wall may depict an elevated protuberance, designated as Rokitansky nodule [1,2].
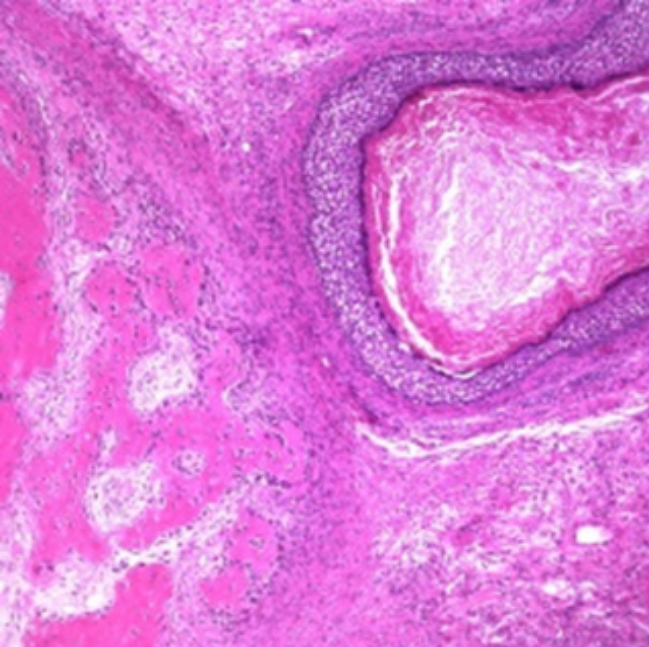 Figure 1: : Mature cystic teratoma depicting an amalgamation of
three developmental layers with foci of squamous epithelium,
cystic cavity imbued with keratin, adipose tissue and fibrous tissue
[5].
Figure 1: : Mature cystic teratoma depicting an amalgamation of
three developmental layers with foci of squamous epithelium,
cystic cavity imbued with keratin, adipose tissue and fibrous tissue
[5].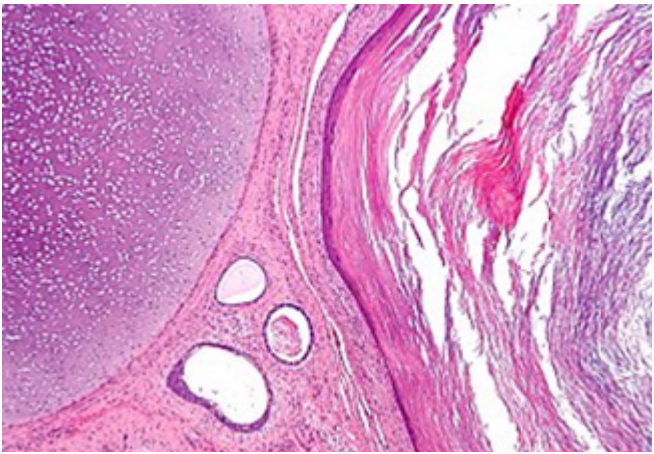 Figure 2: : Mature cystic teratoma exemplifying a fluid-filled cystic
cavity with foci of compressed squamous epithelium, keratin flakes,
fibrous tissue and intervening ovarian stroma [6].
Figure 2: : Mature cystic teratoma exemplifying a fluid-filled cystic
cavity with foci of compressed squamous epithelium, keratin flakes,
fibrous tissue and intervening ovarian stroma [6].Frozen section is exceptionally performed and exemplifies an admixture of mature soft and bony tissues engendered from diverse developmental layers. Solid areas necessitate thorough tissue sampling in order to exclude the occurrence of an immature teratoma. Upon microscopy, commingling of various mature, benign tissues is encountered. Frequently delineated ectodermal tissues appear as squamous epithelium, sebaceous glands, hair follicles or brain tissue. Mesodermal tissues are constituted of bone, cartilage, smooth muscle, fibrous tissue or adipose tissue. Endodermal tissue is exceptionally discerned and composed of intestinal or respiratory epithelium, thyroid or salivary gland. Microscopic foci of immature neuro-epithelium ≤4 foci appear non- indicative of immature teratoma. Foci of adipose tissue necrosis or foreign body reaction may be discerned. Tumefaction associated with Anti–N-Methyl-DAspartate Receptor (NMDAR) encephalitis exhibits neuroglial tissue along with lymphoid tissue aggregates with germinal centres, minimal mature neurons and hyper-cellular foci of astrocytes [1,2].
Mature teratoma necessitates a segregation from neoplasms such as immature teratoma, monodermal teratoma representing as neuro-ectodermal cyst, epidermoid cyst, struma ovarii or strumal carcinoid, endometrial carcinoma, ovarian sarcoma, sex cord/stromal tumour, ovarian germ cell tumour or Krukenberg tumour. Additionally, distinction is mandated from conditions such as ectopic pregnancy, haemorrhagic ovarian cyst, tuboovarian abscess, pedunculated fibroid, polycystic ovary, simple cyst, endometrioma or cystadenoma. Non-gynaecological conditions requiring demarcation emerge as renal cyst, peritoneal cyst, bladder diverticulum, pelvic kidney, peritoneal inclusion cyst, diverticular abscess, peritoneal or retroperitoneal abscess, retroperitoneal sarcoma, lymphoma, distant metastasis into pulmonary parenchyma or gastrointestinal malignancies. Mature teratoma can be appropriately discerned with ultrasonography or a cogent histological examination [3,4]. Upon ultrasonography, tumour exemplifies shadowing echodensity within the cyst wall, an admixture of mature adipose tissue, hair, cellular debris, anechoic sebum superimposed upon hyperechoic debris and intra-cystic, floating, hyperechoic globules. Computerized Tomography (CT) and Magnetic Resonance Imaging (MRI) depicts a mural thickening, cystic configuration, tooth or a bridge or spherical structure spanning the neoplasm. Fat-fluid or fluid-fluid levels may be observed. Magnetic Resonance Imaging (MRI) delineates enhanced signal intensity upon T1 or T2 weighted imaging and decimated signal intensity upon fat-suppressed T1 weighted imaging. Polypoid tumefaction protruding into cyst cavity with an intrinsic pattern simulating a palm tree may be discerned [3,4].
Surgical eradication of ovarian teratoma is an optimal and recommended therapeutic strategy. Incriminated females within reproductive age group can be appropriately treated with cystectomy for preservation of fertility. Elderly subjects can be subjected to salpingo-oophorectomy. Essentially a benign tumefaction, malignant metamorphosis is exceptional and observed in elderly subjects. Commonly, squamous cell carcinoma is exemplified wherein prognostic outcomes are contingent to stage of disease. Re-classification of tumefaction as immature teratoma upon discernible microscopic foci of neuro-epithelium is unwarranted and prognostic outcomes remain unaltered. Similarly, prognostic outcomes remain unmodified with occurrence of gliomatosis peritonei or mature glial tissue implanted upon peritoneal surfaces.
Emergence of Anti–N-Methyl-D-Aspartate Receptor (NMDAR) encephalitis is associated with superior prognosis, especially with institution of preliminary treatment as immunotherapy or neoplastic excision.
