 Journal of Obstetrics and Gynecological Surgery
A PLATFORM FOR SCIENTIFIC INQUIRY IN THE FIELD OF OBSTETRIC AND GYNECOLOGY FIELD
Journal of Obstetrics and Gynecological Surgery
A PLATFORM FOR SCIENTIFIC INQUIRY IN THE FIELD OF OBSTETRIC AND GYNECOLOGY FIELD
 Journal of Obstetrics and Gynecological Surgery
A PLATFORM FOR SCIENTIFIC INQUIRY IN THE FIELD OF OBSTETRIC AND GYNECOLOGY FIELD
Journal of Obstetrics and Gynecological Surgery
A PLATFORM FOR SCIENTIFIC INQUIRY IN THE FIELD OF OBSTETRIC AND GYNECOLOGY FIELD
Lokossou AG Gatien1,2*, Davito Lucien1, Badarou Amyrath1, Accrombessi Manfred3,4, Azonnakpo Julien2, Kounoudji Giscarde2, Lozes E Josette Marie2, Dossou-Gbété Lucien6, Hounkpatin Benjamin1, Perrin René-Xavier1, and Barbeau Benoit5*
1Centre Hospitalier et Universitaire de la Mère et de l’Enfant de la Lagune (CHU-MEL), Cotonou, Bénin
2École Polytechnique d’Abomey Calavi (EPAC), Abomey-Calavi, Bénin
3Fondation pour la Recherche Scientifique (FORS), Cotonou, Bénin
4Faculty of Infectious and Tropical Diseases, Disease Control Department, London School of Hygiene and Tropical Medicine, WC1E 7HT London,
United Kingdom
5Université du Québec à Montréal (UQAM), Département des sciences biologiques and Centre d’excellence en recherche sur les maladies orphelines-Fondation Courtois (CERMO-FC), Montréal, Canada
6Clinique Louis Pasteur, Porto-Novo, Bénin
Correspondence to: Lokossou AG Gatien, Centre Hospitalier et Universitaire de la Mère et de l’Enfant de la Lagune (CHU-MEL), Cotonou, Bénin; E-mail: gatien.
lokossou@epac.uac.bj
Barbeau Benoit, Université du Québec à Montréal (UQAM), Département des sciences biologiques and Centre d’excellence en recherche sur les maladies
orphelines-Fondation Courtois (CERMO-FC), Montréal, Canada; E-mail: barbeau.benoit@uqam.ca
Received date: October 17, 2020; Accepted date: October 28, 2020; Published date: November 4, 2020
Citation: Gatien LAG, Lucien D, Amyrat B, et al. (2020) Low Serum-Derived Syncytin-2 Levels in Exosome at Early Pregnancy is a Predictor of Preeclampsia: A
Prospective Pilot Study in Benin, West Africa. J Obst Gynecol Surg 1(2): pp. 1-6. doi: 10.52916/jogs204010
Copyright: ©2020 Gatien LAG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits
unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Preeclampsia (PE) affects 2 to 8% of pregnant women and represents one of the major causes of maternal and perinatal morbidity and mortality, particularly in sub-Saharan Africa. A limited number of biomarkers have been proposed for the identification of pregnant women predisposed to preeclampsia. Syncytin-2 is an endogenous retrovirus envelope protein playing a key role in placental formation through the fusion of villous cytotrophoblasts, resulting in syncytiotrophoblast formation. The reduction of Syncytin-2 levels detected in placental tissue and on the surface of exosomes has been shown to strongly correlate with the severity of symptoms in preeclamptic patients. We were thus interested in conducting an analysis of a Benin cohort of pregnant women over the predictive value of this marker. From July 2015 to January 2017, 260 pregnant women were recruited in two health facilities. Blood samples were monthly collected from the beginning of pregnancy up to 20 weeks of gestation and exosomes were then isolated. We then compared Syncytin-2 levels in exosome preparations from women who presented PE to those with normal pregnancy. Our results showed that Syncytin-2 significantly decreased between 7 to 10 weeks of gestation in pregnant women with PE compared to normal pregnant women (p=0.02). Our study thereby suggests that Syncytin-2 could be a promising biomarker for early diagnosis of PE.
Pregnancy, Preeclampsia, Early diagnosis, Exosomes, Syncytin-2.
Preeclampsia (PE) is the most common placental disorder occurring during pregnancy worldwide [1]. It is characterized by vascular dysfunction and exacerbated inflammation [2-6]. This disorder is often associated with changes in the levels of cytokines, chemokines, blood coagulation factors, and apoptotic markers [7]. In most cases, PE leads to multi-organ failure in the mother such as renal insufficiency, liver dysfunction, neurological or hematological complications, uteroplacental dysfunction and is responsible for significant maternal and perinatal morbidity and mortality. PE affects 2 to 8% of pregnancies representing a very high burden in low and middle-income countries (LMIC) [8-11]. In sub-Saharan Africa, severe PE and eclampsia are diagnosed in more than 15% of pregnancy [12]. Indeed, 99% of deaths related to PE occur in LMIC [12,13]. Symptoms related to PE result from systemic inflammation, oxidative stress, and endothelial dysfunctions [2]. Severe PE is normally associated with the early appearance of symptoms, but can also occur at a later stage, i.e. 4-12 weeks postpartum [14]. However, suitable management of PE would require the early detection of predisposed pregnant women.
Important progress has been made in understanding the pathophysiology of PE. However, early diagnosis remains limited. Only two biomarkers are being used for the diagnosis of PE: low PlGF (Placental Growth Factor) and high sFlt-1(Soluble Fms-Like Tyrosine Kinase 1). sFlt-1/PlGF ELISA ratio is used in Europe, US, and Canada for the identification of women at risk of developing PE in the second and third trimesters of pregnancy [15,16]. However, earlier diagnosis of PE is crucial in reducing maternal and fetal perinatal morbidity and mortality and preventing long-term postnatal maternal and newborn complications.
Syncytin-2 (S2) is a protein encoded by a human endogenous retrovirus (HERV) gene [17, 18] with fusogenic and immunosuppressive functions [19-22]. In addition to its key role in placental formation through the fusion of villous cytotrophoblasts with the multinuclear syncytiotrophoblast, exosome-associated S2 has also been suggested to contribute to the immunomodulation prevailing in the placental environment [20,21,23-26]. Interestingly, it has been shown that serumderived exosomes and placental tissues from PE patients showed lower abundance in S2 when compared to samples from women with normal pregnancy [25, 27]. We have therefore hypothesized that S2 embedded on the surface of exosomes could be used as an early biomarker of PE. The present study aimed to assess the beneficial predictive value of exosomeassociated S2 in pregnant women predisposed to develop PE. Our results demonstrate a significant difference in S2 levels in PE patients vs. control at early time points and suggest that this protein should be further considered as an early marker for PE predisposition.
Pregnant women (n=533) were recruited in two health facilities in Southern Benin (the Centre Hospitalier Universitaire-Mère et Enfant de la Lagune and the Deo Gratias hospital). The study was conducted from July 2015 to January 2017. Following the exclusion of 273 women due to several reasons, 260 women were maintained in the study and were thus followed throughout their pregnancy with continuous monitoring for PE symptoms based on the recommendation from the International Society for the Study of Hypertension in Pregnancy, the Canadian Hypertension Society, and the Society of Obstetricians and Gynaecologists of Canada and Benin. PE was defined as de novo hypertension detected after 20 weeks of gestation (WG) combined with proteinuria (>300 mg/day) [28]. Only 23 (8.8%) samples were harvested before 7 WG. Among them, 2 (0.77%) had developed PE.
Peripheral blood (7 ml) was monthly harvested from all participants from the beginning of pregnancy to 20 WG. Serum from blood samples was first stored at -20°C. Exosomes were then isolated, using the ExoQuick™ kit (EXOQ20A-1, System Bioscience, Mountain View, CA, USA), as previously described [25]. Briefly, sera were thawed on ice and centrifuged at 3000g for 15 minutes to remove cells and cellular debris. Serum-derived exosomes were isolated by adding exosome precipitation solution to 250 μl of serum. Tubes were inverted three times and the mixture was then incubated for 30 minutes at 4°C. After centrifugation at 1500 g for 30 minutes at 4°C, supernatant and excess of Exoquick solution were gently removed. After 2 consecutive washes in 0.1 M sodium phosphate, the pellet was resuspended in 100 μl binding buffer solution. Exosome preparations were next incubated at 37°C for 20 minutes, and after centrifugation at 1500 g for 5 minutes, supernatants (containing exosomal proteins) were transferred to a new tube and quantified for protein concentration using the PierceTM BCA Protein Assay Kit (2322; Waltham, Ma, USA). Samples were then stored at -20°C until use.
The CD63 ExoELISA kit (EXOEL-CD63A-1; System Biosciences) was used to normalize the amount of exosomes from sera, according to manufacturer’s recommendations and further optimized for S2 quantification. Briefly, 96 well plates were incubated overnight at 37°C with 50 μg of exosomal proteins. Plates were washed three times with phosphate-buffered saline (PBS) and incubated with anti-CD63 (1:100; System Biosciences) or our purified anti-Syncytin-2 antibody (4 μg/ml in blocking solution [25]). Bound anti-CD63 or anti-Syncytin-2 antibodies were then detected with horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (1:5000; System Biosciences) added for 1 h at room temperature. After three washes, HRP activity was assessed by the addition of the TMB (TetraMethylBenzidine) substrate. After the addition of the stopping solution (H2SO4 1M), absorbance (450 nm) of each sample was measured on a microplate reader (F 200 pro Tecan). S2/CD63 ratios were finally determined from the measured absorbances.
Data were collected and analyzed using Microsoft Excel 2010 and Stata 15 (College Station, TX, USA), respectively. Mean and proportion was compared using paired Student t, Mann Whitney and Chi2 Pearson, or Fisher exact test depending on the distribution of variables. The threshold of significance was set to a p-value < 0.05. The median of the ratios of S2/CD63 was outlined using quartile values.
This study was approved by the Ethics Committee from the Institute of Applied Biomedical Science of Cotonou, Benin. All recruited pregnant women had signed an informed consent form to participate in the study.
The mean age in our study group was 28.70 (SD ± 5.13, range 15-41) years (Table 1). The mean age in the PE group was 27.09 SD ± 6.24) years in comparison to 27,98 (SD ± 5,22) years in the normal pregnant women group. The mean gestational age at delivery was 38.70 WG (SD ± 1.52) in normal pregnant women and 37.76 WG (SD ± 2.07) in PE women. All PE patients (n=24, 8%) had diastolic blood pressure exceeding 90 mmHg and proteinuria < 0.3 g/d (in 24-hour harvest) Thirty-one percent of the women were primiparous gravidity.
Table 1: Clinical characteristics of healthy pregnant women and patients with preeclampsia| Characteristics | Healthy pregnant women (n=236) | PE (n=24) | P-value |
|---|---|---|---|
| Age | 27.98 ± 5.22 | 27,09 ± 6.24 | 0.435 |
| Gestational ages at delivery | 38.70 ± 1.52 | 37.76 ± 2.07 | 0.058 |
| Age and gestational ages at delivery are expressed as means ± SD. | |||
S2 and CD63 levels were quantified by ELISA in serum-derived exosomal samples and the logarithm values of S2/CD63 ratios were then calculated. In the overall study population, the incorporation of the S2/CD63 ratio was constant between 0 and 20 weeks of gestation (Figure 1A). Although S2/CD63 ratios in serum-derived exosomes of normal pregnant women showed the same trend (Figure 1B), in PE patients, ratios were more modest, especially at early time points (Figure 1C).
 Figure 1:CD63-normalised S2 levels in serum-derived exosomes of pregnant women according to gestational age. S2 and CD63 levels were measured in serumderived exosomes from normal and PE pregnant women by ELISA from the first to the 20th gestational week. The natural logarithm (ln) mean values of S2/CD63
ratio were calculated and are presented as a solid line for the entire cohort, (A): healthy pregnant women (B): and PE patients (C): IC95% values are presented
as dashed lines. Circles represent each patient.
Figure 1:CD63-normalised S2 levels in serum-derived exosomes of pregnant women according to gestational age. S2 and CD63 levels were measured in serumderived exosomes from normal and PE pregnant women by ELISA from the first to the 20th gestational week. The natural logarithm (ln) mean values of S2/CD63
ratio were calculated and are presented as a solid line for the entire cohort, (A): healthy pregnant women (B): and PE patients (C): IC95% values are presented
as dashed lines. Circles represent each patient.The mean of the S2/CD63 ratio in serum-derived exosomes harvested before 10 weeks of gestation, showed higher levels of S2 in samples from normal pregnant women compared to those with PE (P=0,04)(Table 2). Women with a ratio of S2/CD63 ≤ 17.5 before 10 weeks of gestation were 6 times more likely to suffer from preeclampsia (P=0,03)(Table 3).
Table 2: Measurement of S2/CD63 ratios in normal pregnant women vs. PE patients at 10 weeks of gestation.| Status | Observed | Mean of | P-value |
|---|---|---|---|
| Control | 74 | 30.63 ± 27.80 | 0.04 |
| Preeclampsia | 10 | 12.55 ± 11.68 | |
| Total | 84 | 28.47 ± 27.00 |
| S2/CD63 | Odd Ratio | 95% Conf Interval | P-value |
|---|---|---|---|
| >17.5 | 1 | ||
| < 17.5 | 5.87 | 1.16-29.57 | 0.032 |
Pregnant women were further analyzed according to their gestational age. As depicted in Figure 2, an increased abundance of S2 was observed in serum-derived exosomes from normal pregnant women vs. PE women between 0 and 13 WG.
To determine the useful time for an early diagnosis of PE, we compared the median of the S2/CD63 ratio at different gestational ages. The median distribution of S2/CD63 ratios varied with gestational age and preeclampsia status with a significant difference displayed before 10 WG (P=0.03) and between 10 to 13 (P=0.04) weeks of gestation between norma and PE-derived samples (Figure 2). Specifically, the median S2/ CD63 ratio between 7 and 10 WG among women with PE was significantly lower than those with normal pregnancy (P=0.02) (Figure 3).
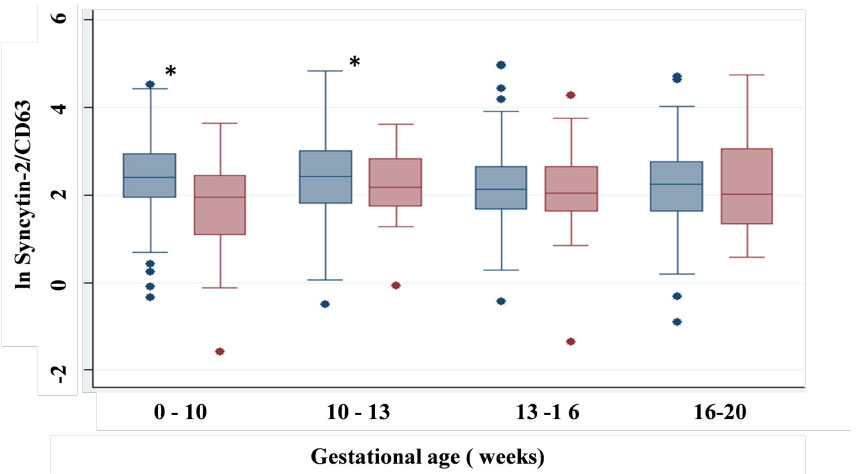 Figure 2: Comparison of the median of S2/CD63 ratios in relation to the gestational age (weeks) and PE status. Values represent the mean of S2/CD63 ratios
in normal pregnant women (pale blue) and in PE patients (pale red). Median values varies with gestational age and PE status, showing a significant difference
between 0-10 (P=0.03) and 10-13 (P=0.04) weeks of gestation.
Figure 2: Comparison of the median of S2/CD63 ratios in relation to the gestational age (weeks) and PE status. Values represent the mean of S2/CD63 ratios
in normal pregnant women (pale blue) and in PE patients (pale red). Median values varies with gestational age and PE status, showing a significant difference
between 0-10 (P=0.03) and 10-13 (P=0.04) weeks of gestation.  Figure 3: Low levels of CD63-normalised S2 from 7 to 10 weeks of gestation in
serum-derived exosomes from PE patients. Values represent the mean of S2/
CD63 ratios in normal pregnant women (blue) vs. PE patients (red). Median
S2/CD63 values in women with PE is significantly lower than in pregnant
women from the control group between 7 and 10 WG (P=0.02).
Figure 3: Low levels of CD63-normalised S2 from 7 to 10 weeks of gestation in
serum-derived exosomes from PE patients. Values represent the mean of S2/
CD63 ratios in normal pregnant women (blue) vs. PE patients (red). Median
S2/CD63 values in women with PE is significantly lower than in pregnant
women from the control group between 7 and 10 WG (P=0.02). We aimed to assess the potential relevance in using S2 as an early biomarker of PE. In this study, we showed that S2 levels significantly decreased from 7 to 10 weeks of gestation in serum-derived exosomes in pregnant women with PE compared to normal pregnant women (p=0.02). Our data thus suggests that S2 could be a promising biomarker for early diagnosis of PE.
As clinical signs of PE only appear at 20 weeks of gestation, diagnosis of PE before this period of gestation should be beneficial for appropriate medical monitoring of suspected cases of PE. To determine the most adequate time point, we have compared the incorporation of in serum-derived exosomes of healthy pregnant women with those of PE patients before 10 weeks of gestation. Recruitment of pregnant women during the first trimester of gestation especially before 10 WG was an important challenge, as most Beninese women come for their first antenatal consultation between 16 and 20 WG. Nonetheless, we were successful in recruiting several pregnant women before 10 weeks of gestation.
Several studies have shown that preeclampsia is associated with abnormal placentation [2]. This defect is followed by a systemic inflammatory response [2-5]. Also, as the placenta produces exosomes with immunosuppressive properties [21,23], potential defects in exosomes could also contribute to the severity of PE symptoms [21,25]. Further evidence from our group and others have argued that HERV-encoded S2, known to be implicated in the formation of the placenta and the maintenance of the immunosuppressive environment [20-22,29,30], shows a marked reduction in its levels in exosome and placental tissue of PE patients. These observations suggested that S2 could be used for PE diagnosis, especially knowing that serum-derived exosomes produced by the placenta should reflect the level of S2 expressed in the placenta.
Isolation of exosomes from blood represents a very useful choice for diagnostic purposes. However, many factors such as the type of anticoagulant, blood handling, time of sample collection, hemolysis, and others, may affect the composition and characteristics of exosomes in downstream analysis, leading to inaccurate results [31]. Thus, to ensure the quality of placenta-derived exosomes, the International Society for Extracellular Vesicles has recommended the use of tetraspanins (CD9, CD63, and CD81 and HSP70) to define exosomes [32].
In this study, we have used CD63 to normalize the number of exosomes, although we are aware that this marker is also present in other subpopulations of extracellular vesicles [33].
Our data show reduced S2/CD63 levels at early time points. This threshold is close to level 3, the natural logarithm value of the normal S2/CD63 ratio. When it is close to level 2, the risk of PE becomes high (Figure 1). Our data showed that S2/CD63 ratios during the time frame of 0-10 and 10-13 weeks of gestation was significantly lower in women who had developed PE during their pregnancy compared to women with normal pregnancy (p=0.04).The S2/CD63 ratio in women with PE was still significantly lower than in the control group of pregnant women between 7 and 10 weeks of gestation. We had very few samples before 6 weeks of gestation (n=6) and no cases of PE during or after pregnancy, which did not allow us to address the reliability of our marker during this time frame. Noteworthy, our results further confirm our previous studies showing lower incorporation of S2 on the surface of serum-derived exosomes of women with PE [25].
As PE is a multifactorial pathology, a single marker is likely not sufficient in terms of diagnostic value [8]. Many other markers have been assessed, such as the anti-angiogenic factor sFlt-1 released by the placenta, which is involved in the endothelial dysfunction observed in PE. In women with PE, the levels of sFlt1 rise 5 weeks before clinical detection and stay elevated until the onset of PE [34]. However, measurement of sFlt-1 between 11 and 13 weeks of gestation did not improve the prediction of PE [35]. sEndoglin has also been proposed as a suitable marker to assess the severity of PE and has been associated with an increased risk of adverse outcomes [36]. An established risk model based on VEGF, sEndoglin, PlGF, and sEGFR levels in serum has been tested and showed to predict PE at the late stage of pregnancy, but unfortunately not at early stages [37].
To improve early PE diagnosis, multiparametric models including biochemical, molecular, and genetic markers, are direly needed to provide the most reliable and accurate combination of markers for early diagnosis of PE [38-43]. Our data indicate that S2 remains a suitable marker for early diagnosis of PE and should be tested in combination with other current markers.
Significant progress has been made in the understanding of the pathophysiology of PE and suggests that the best predictive value for PE development during pregnancy should be offered by a combination of markers. In this study, we showed that exosome-associated S2 measured between 7 and 10 weeks of gestation. is reduced in PE patients when compared to normal pregnant women. Our data thereby suggest that S2 is a promising marker for early PE diagnosis. Further multicenter studies and multivariate analyses will be needed to validate its use
The authors thank all pregnant women and midwives, from CHU-MEL and Clinique Déo gratias, who participated in this study. This work was supported by the Canadian International Development Research Centre (BB) (grant no. 107718- 00020699-027), Canadian Institutes of Health Research (BB) (grant no. MOP301784), March of Dimes Foundation (BB) (grant no. 6-FY13-155), and The World Academy of Science (AGGL) (grant no. 15-062 RG/BIO/AF/AC).
